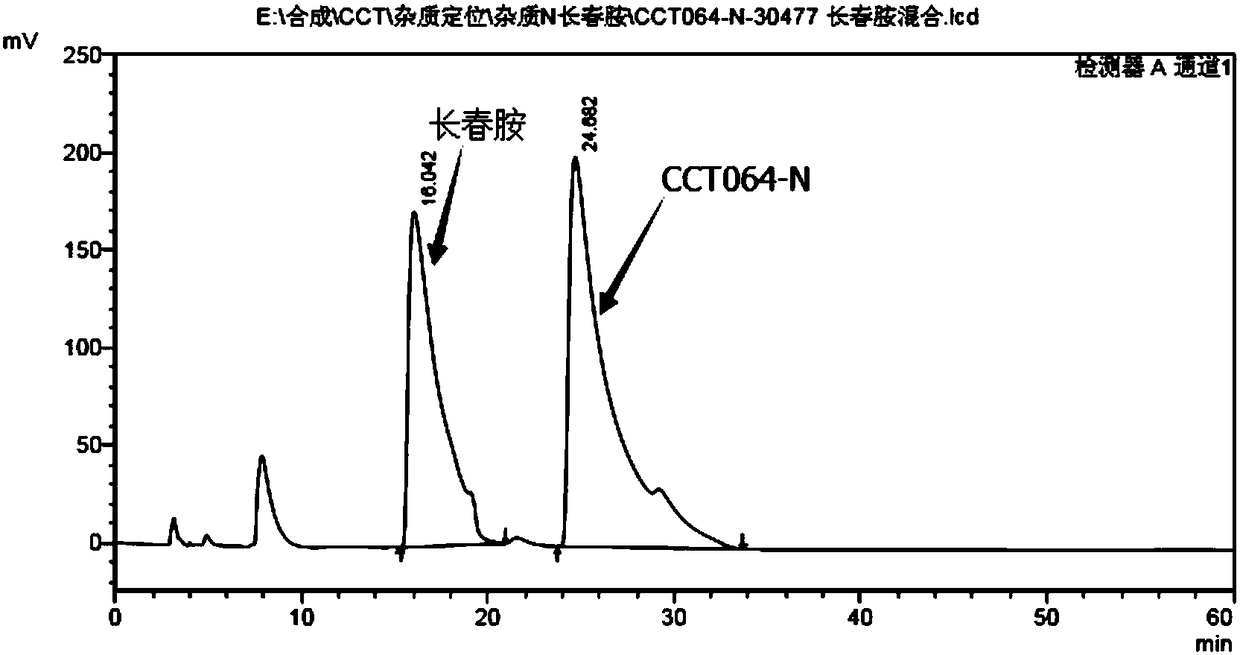
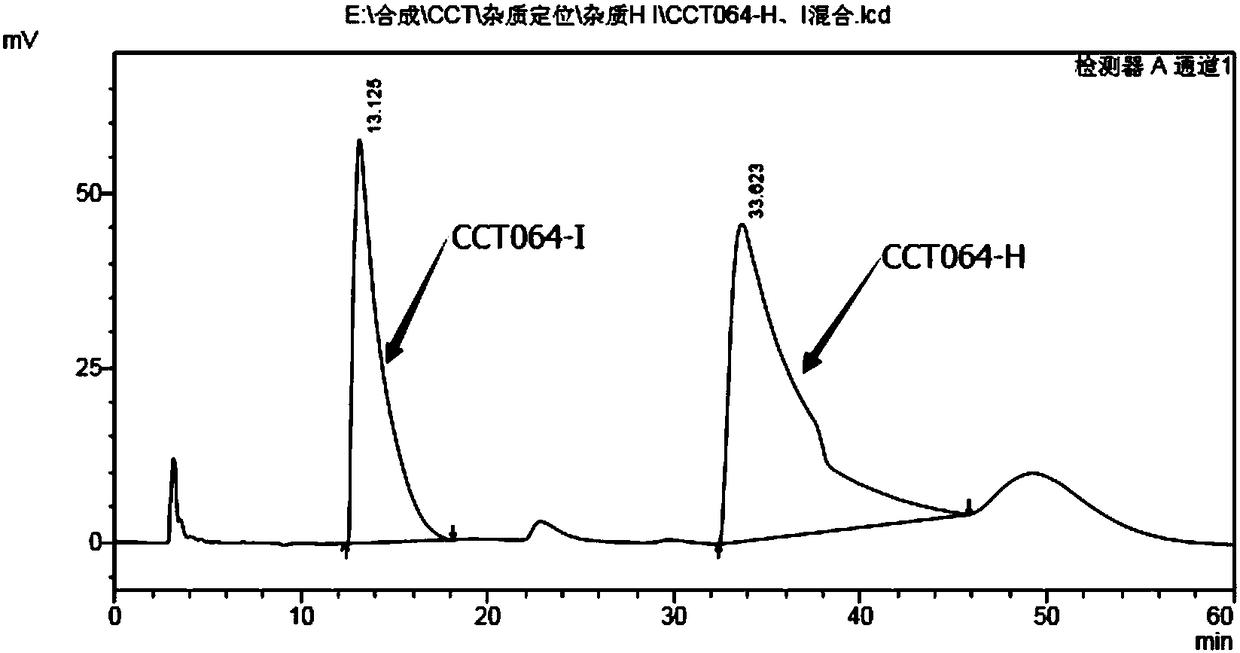
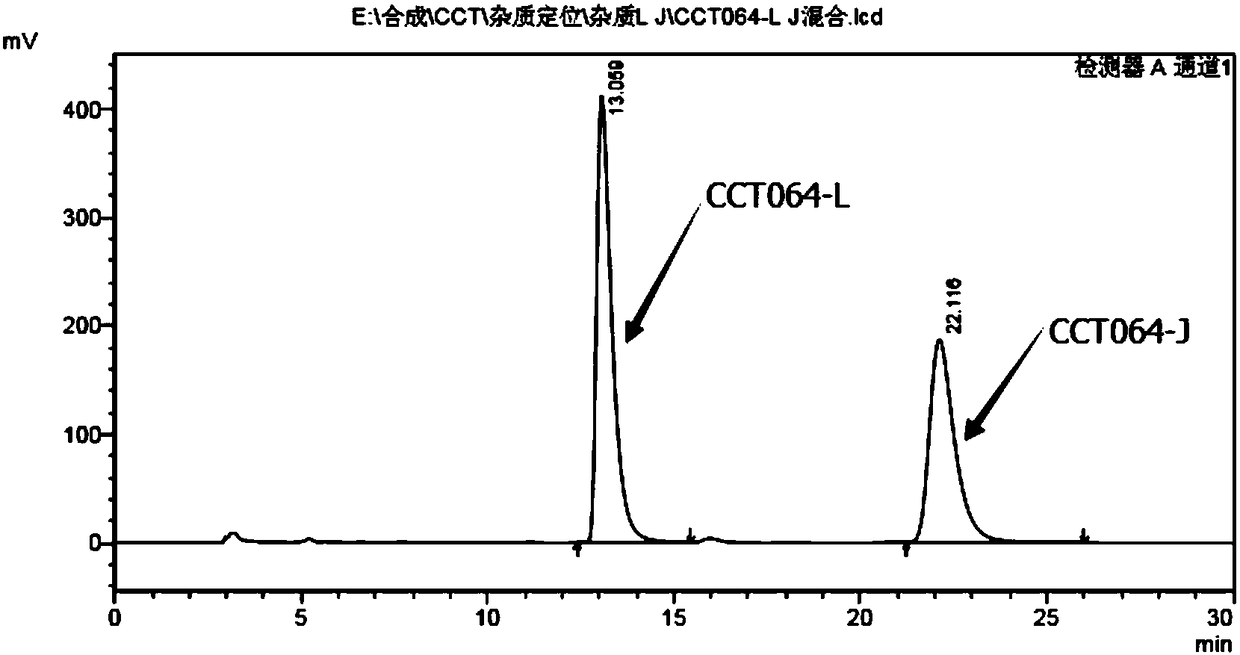
Preparation methods for chiral impurities of vincamine
A technique for vincamine and chiral isomers, which is applied in the field of preparation of chiral impurities in vincamine, can solve the problems such as no seven preparation methods of chiral impurities reported in the literature, and achieves simple preparation steps and post-processing, and mild reaction conditions. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] 1.1, (4aR,15bR)-4a-ethyl-4a,5,6,7,10,15-hexahydro-2H,9H-indolo[2,3-a]pyrano[3,2- i] quinozine-3,3(4H)-dicarboxylate and (4aS,15bS)-4a-ethyl-4a,5,6,7,10,15-hexahydro-2H,9H-indolo Preparation of [2,3-a]pyrano[3,2-i]quinazine-3,3(4H)-dicarboxylate (CCT064-A-106(1)&CCT064-A-107)
[0082] Add CCT064-A-105 (216g, 0.476mol, 1.0eq), ethanol (2.16L), dichloromethane (DCM) (110mL) into a 3L three-necked flask, heat the oil bath to 30°C and add L-dibenzoyl Tartaric acid hydrate (178.8g, 0.473mol, 1.0eq) was added and stirred for about 1h. The reaction solution was filtered, the filter cake was rinsed with ethanol (300 mL), and dried at 40° C. to obtain CCT064-A-106 (236 g, 61%) as a yellow solid.
[0083] The collected filtrate was concentrated to dryness at 40°C under reduced pressure to obtain a yellow foamy solid (250g), which was added with dichloromethane (500mL), and 10% potassium carbonate aqueous solution (500mL) was stirred and separated, and the aqueous pha...
Embodiment 2
[0120]
[0121] 2.1, 2-(((1R,12bR)-1-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinazine-1- Base) the preparation of methyl) diethyl malonate (CCT064-F-101)
[0122] Add CCT064-A-106(1) (55.2g, 0.12mol, 1.0eq), N,N dimethylformamide (DMF) (220mL, 4vol) into a 500mL three-necked flask, stir magnetically at room temperature, and wait until the reaction solution dissolves After clearing, add palladium carbon (5.5g, 0.1w / w) and replace with hydrogen three times. After about 4 hours of reaction, TLC (DCM / MeOH=20 / 1) shows that the raw material has reacted by 1 / 2. Filter the reaction solution and replace the palladium carbon , continue to react for about 5h, TLC raw material reaction is complete. The reaction solution was filtered to remove palladium carbon, and the filtrate was added with concentrated ammonia water (55 mL) and stirred for 1 h. Then add water (600mL) to the reaction solution, stir ethyl acetate (200mL) thoroughly, separate the liquids, extract the aqueous pha...
Embodiment 3
[0153]
[0154] 3.1, 2-(((1S,12bR)-1-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinazine-1- Base) the preparation of methyl) diethyl malonate (CCT064-G-101)
[0155] Add CCT064-A-107 (20g, 0.044mol, 1.0eq), ethanol (300mL, 15vol) into a 1L three-neck flask, stir magnetically at room temperature, then add glacial acetic acid (10.8mL, 0.3vol) and stir for 10min, then add in batches Sodium borohydride (3.35g, 0.088mol, 2.0eq), stirred for 10min after addition, added concentrated ammonia water (8mL, 0.4vol), moved to an oil bath and heated to 50°C, reacted for about 1h, TLC (DCM / MeOH=20 / 1) shows that the raw material has reacted completely. The reaction solution was cooled to room temperature, concentrated under reduced pressure to remove the solvent to obtain 28.8 g of a yellow solid, which was added with dichloromethane (300 mL), and water (200 mL) was stirred and separated, and the aqueous phase was extracted three times with dichloromethane (200 mL×3), and combined ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com