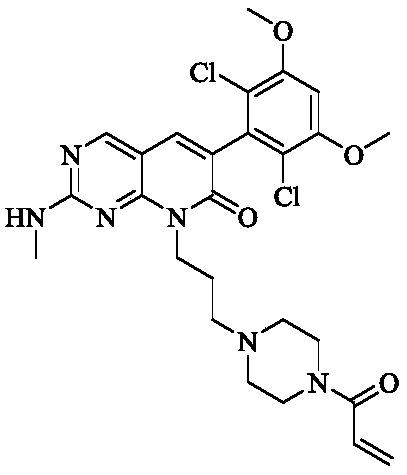
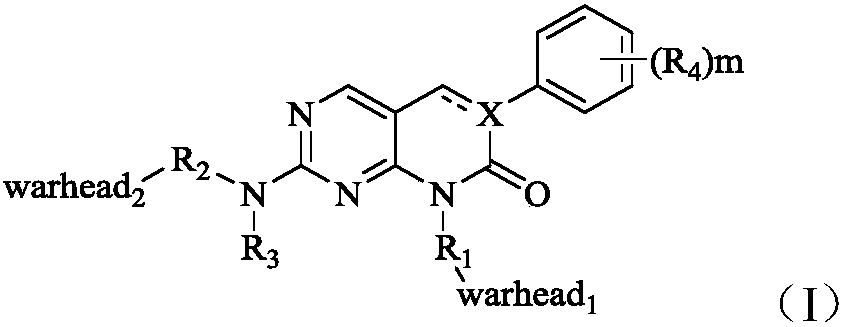
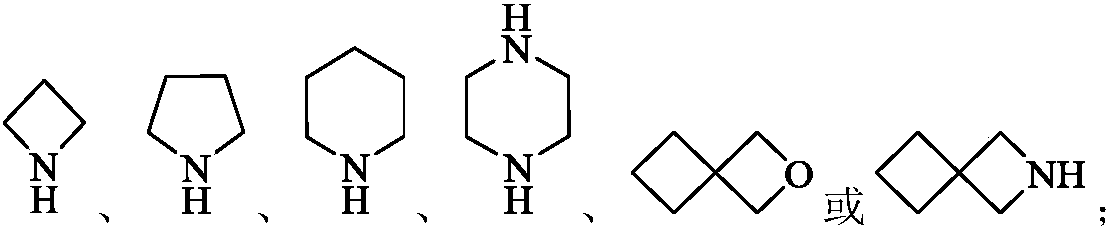
Fibroblast growth factor receptor inhibitors and application thereof
A growth factor receptor, fibroblast technology, applied in the field of medicine, can solve the problems of not strong and durable drug effect, easy to induce drug resistance, and not good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0310] The abbreviation "NMP" used herein refers to N-methylpyrrolidone; "DIPEA" refers to N,N-diisopropylethylamine; "TLC" refers to thin layer chromatography; "PE:EA" refers to petroleum ether : ethyl acetate; "TFA" refers to trifluoroacetic acid; "THF" refers to tetrahydrofuran; "EA" refers to ethyl acetate; "DCM:MeOH" refers to dichloromethane:methanol; "DCM" refers to dichloromethane Methane; "MTBE" refers to methyl tert-butyl ether; "TFAA" refers to trifluoroacetic anhydride; "TEA" refers to triethylamine; Butoxycarbonyl; "MsCl" is methanesulfonyl chloride; "DEAD" means diethyl azodicarboxylate; "PPh 3 " refers to triphenylphosphine; "mCPBA" refers to m-chloroperoxybenzoic acid; "LC-MS" refers to liquid chromatography-mass spectrometry.
[0311] The present invention can be better understood from the following examples and experimental examples. However, those skilled in the art can easily understand that the content described in the embodiments is only for illustrating ...
preparation example 1
[0312] Preparation example 1: the synthesis of intermediate 4-amino-2-(methylthio)pyrimidine-5-carbaldehyde
[0313]
[0314] Step 1: Synthesis of ethyl 4-amino-2-(methylthio)pyrimidine-5-carboxylate
[0315]
[0316] The raw material 4-chloro-2-(methylthio)pyrimidine-5-carboxylic acid ethyl ester (15g, 64.4mmol, 1.0eq) was dissolved in tetrahydrofuran (100mL), and triethylamine (19.6g, 193.4mmol, 3.00eq ), cooled to 0°C, slowly added ammonia water (7.8g, 1288mmol, 20.0eq), raised to room temperature and reacted overnight, LC-MS detected that the reaction was complete, left to separate the liquids, extracted the aqueous phase once with ethyl acetate, combined The organic phase was dried and concentrated, and the crude product was slurry-washed with methyl tert-butyl ether to obtain a white solid product (11.8 g, yield: 87%).
[0317] Step 2: Synthesis of (4-amino-2-(methylthio)pyrimidin-5-yl)methanol
[0318]
[0319] The raw material 4-amino-2-(methylthio)pyrimidin...
preparation example 2
[0323] Preparation Example 2: Synthesis of intermediate 6-((methylsulfonyl)oxy)-2-azaspiro[3.3]heptane-2-carboxylic acid tert-butyl ester
[0324]
[0325] Step 1: Synthesis of tert-butyl 6-((methylsulfonyl)oxy)-2-azaspiro[3.3]heptane-2-carboxylate
[0326]
[0327] The raw material tert-butyl 6-hydroxy-2-azaspiro[3.3]heptane-2-carboxylate (1.1g, 5mmol, 1.0eq) was dissolved in dichloromethane (15mL), triethylamine (1.5g, 15mmol, 3.0eq) and methanesulfonyl chloride (573mg, 5mmol, 1.0eq), react at room temperature for 2 hours. LC-MS detected that the reaction was complete, the reaction solution was poured into water, extracted with dichloromethane, the organic phases were combined, washed twice with saturated brine, dried, and concentrated to give a white solid product (1.2 g, yield: 82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com