Goserelin sustained release microsphere freeze-dried powder and preparation method thereof
A slow-release microsphere, freeze-dried powder technology, applied in freeze-dried delivery, powder delivery, microcapsules and other directions, can solve the problems of complex steps, serious burst release, etc., and achieve shortened peak time, high specificity, and convenient medication. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
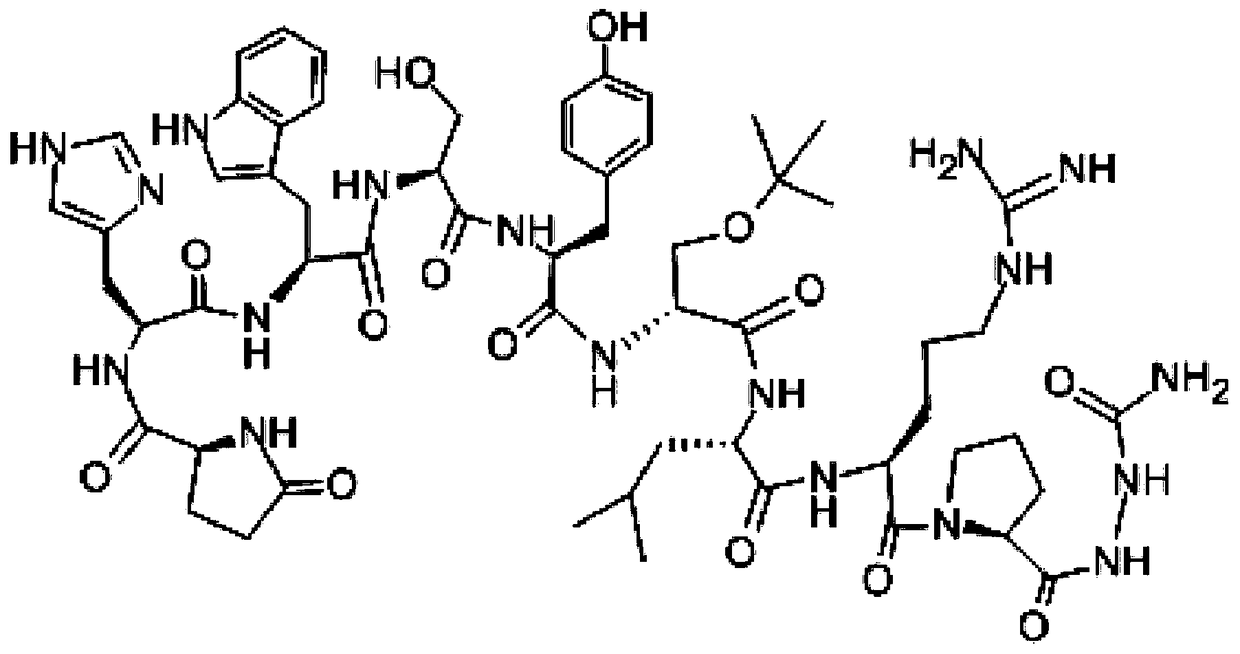
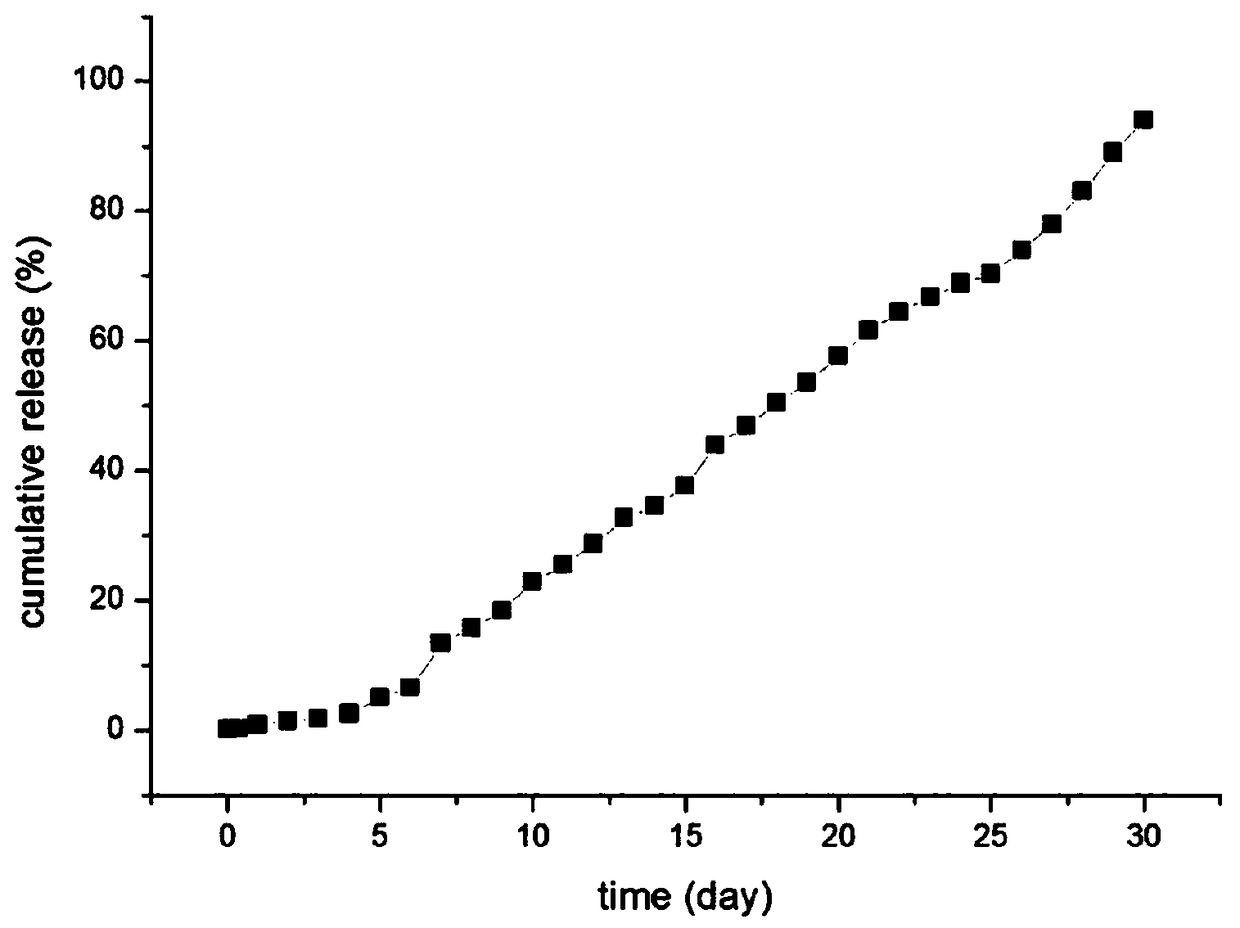
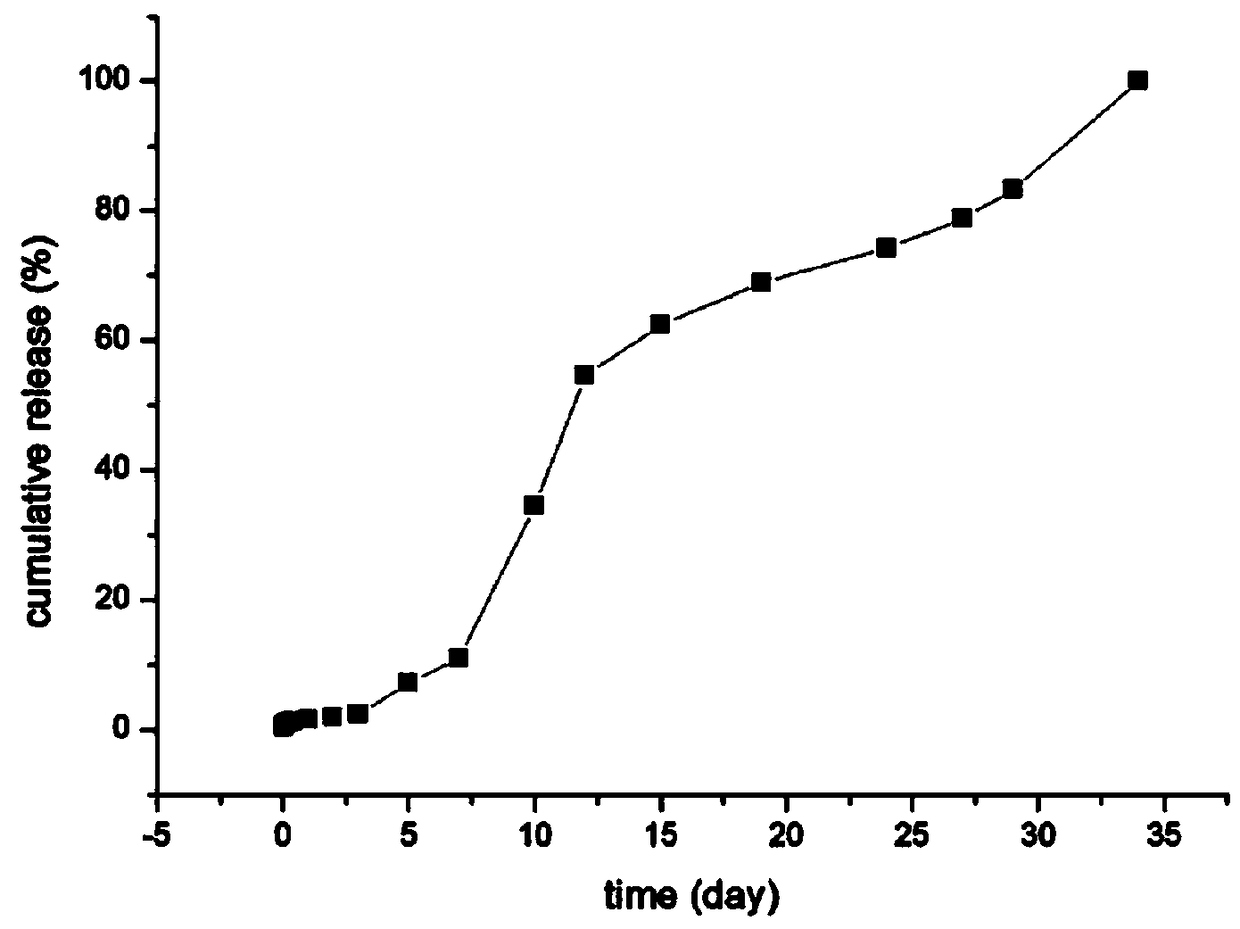
[0042] Weigh 100mg goserelin acetate, 150mg poloxamer 188, 50mg polyethylene glycol 4000 dissolved in 0.6mL water, the HLB value of poloxamer is 11, swell at 4 degrees Celsius for 10 hours to form a gel, As drug solution A. Weigh 0.8 g of lactide-glycolide copolymer (molecular weight: 5000 Dalton) and dissolve it in 5 mL of dichloromethane as solution B. Drug solution A was added to solution B, and colostrum was formed by high-speed shearing in an ice-water bath. The high-speed shearing speed was 12,000 rpm for 3 minutes. The shearing speed is 15000rpm, and the time is 4min. The double emulsion was poured into 200mL 5% NaCl solution, distilled under reduced pressure at 40°C for 30min, centrifuged, washed three times with water, and freeze-dried for 24 hours to obtain microspheres with a particle size of 20-90μm. 200 mesh, 400 mesh and 500 mesh combined standard sieve to obtain microsphere particle size are all within the limit of 25-74 μm, use 60 Sterilized by Co-gamma rays...
Embodiment 2
[0049] Weigh 120mg of goserelin acetate, 110mg of poloxamer 188, 50mg of poloxamer 407, dissolve in 0.9mL of water, the HLB value of poloxamer is 5, swell at 5 degrees Celsius for 8 hours to form a gel , Soluble in A as a drug. Weigh 1.2g of lactide-glycolide copolymer (molecular weight: 10,000 Daltons) and dissolve it in 5mL of dichloromethane as solution B. Drug solution A was added to solution B, and colostrum was formed by high-speed shearing in an ice-water bath. The high-speed shearing speed was 10,000 rpm, and the time was 4.6 minutes. The high-speed shearing speed is 20000rpm, and the time is 2min. The double emulsion was poured into 200mL 2% NaCl solution, distilled under reduced pressure at 70°C for 10min, centrifuged, washed three times with water, and freeze-dried for 24 hours to obtain microspheres. 200 mesh, 300 mesh and 500 mesh combined standard sieve to obtain microsphere particle size are all within the limit of 25-74 μm, use 60 Co-gamma ray sterilization,...
Embodiment 3
[0056] Weigh 200mg of goserelin acetate, 170mg of poloxamer 237, 20mg of poloxamer 228 dissolved in 1mL of water, the HLB value of poloxamer is 20, swell at 2 degrees Celsius for 12 hours to form a gel, as Drug solution A. Weigh 1.6g of lactide-glycolide copolymer (molecular weight: 30000 Daltons) and dissolve in 5mL of dichloromethane as solution B. Drug solution A was added to solution B, and colostrum was formed by high-speed shearing in an ice-water bath. The high-speed shearing speed was 6000 rpm for 5 minutes; The cutting speed was 17000rpm, and the time was 2.5min; the double emulsion was poured into 200mL 3% NaCl solution, distilled under reduced pressure at 35°C for 20min, centrifuged, washed three times with water, and freeze-dried for 24 hours to obtain microspheres. 90 μm, the freeze-dried microspheres passed through 200 mesh, 250 mesh and 500 mesh combined standard sieves to obtain microspheres with a particle size within the limit of 25-74 μm. 60Sterilized by C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com