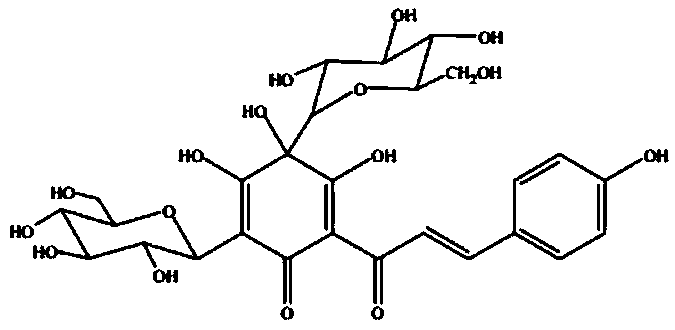
Application of hydroxyl carthamin yellow A in preparation of drugs for treating pyaemia
A technology of hydroxy safflower and yellow pigment, applied in the field of medicine, can solve the problems of putting specific drugs into clinical practice, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 Hydroxysafflower yellow A mouse intravenous administration acute toxicity test
[0030] 1. Experimental materials
[0031] Clean grade SD rats (half male and half female), 180-220g, hydroxysafflor yellow A, physiological saline (0.9% sodium chloride injection).
[0032] 2. Experimental method
[0033] 40 animals (half male and half male) were screened according to animal weight gain, diet, and activity during the adaptation period to enter this experiment, and were divided into 2 groups by weight block method, with 20 animals in each group, half male and half male. The maximum dosage method was used in the experiment, the maximum concentration of hydroxysafflower yellow A was 50mg / mL, and the intravenous injection limit for rats was 6mL / kg. In summary, the maximum dosage was 300mg / kg. The experimental group was given a single administration, 6 mL / kg hydroxysafflower yellow A liquid (50 mg / mL), and slowly injected intravenously; the control group was given...
Embodiment 2
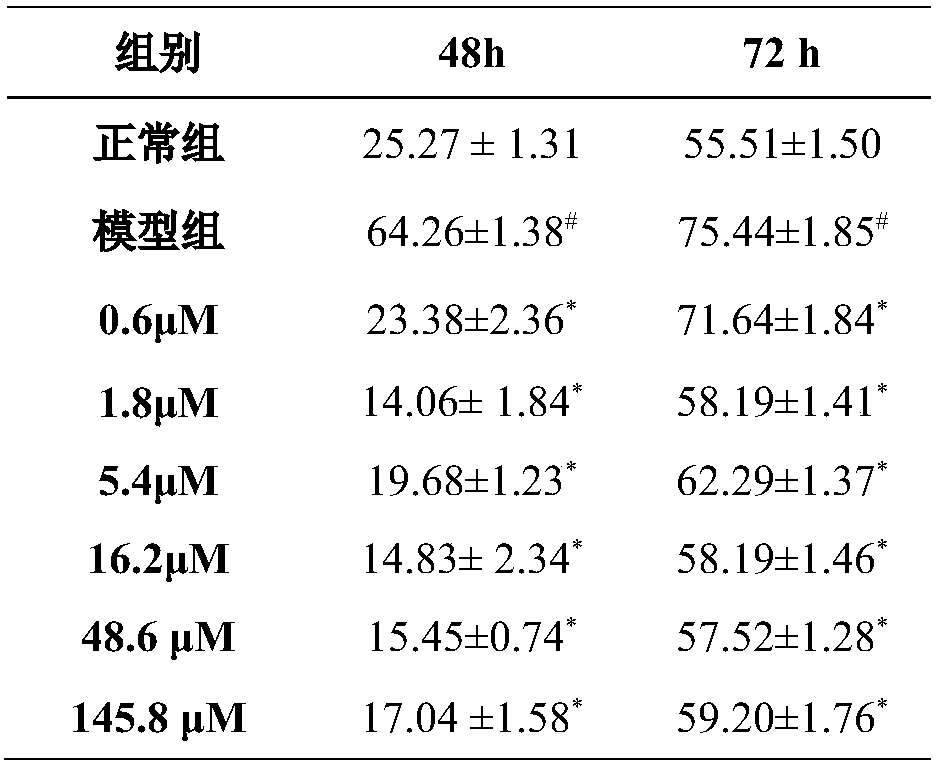
[0038] Example 2. Evaluation of the effect of hydroxysafflor yellow A on the release of HMGB1 from rat peritoneal macrophages stimulated by LPS
[0039] 1. Experimental materials
[0040] Male clean grade SD rats, 180-220g, endotoxin (LPS), hydroxysafflor yellow A, RPMI-1640 medium, 24-well plate, HMGB1ELISA kit.
[0041] 2. Experimental method
[0042]Isolate peritoneal macrophages of male SD rats (male SD rats were fasted for 12 hours before operation, opened the abdominal cavity after anesthesia, injected 10 mL of pre-cooled PBS solution into the abdominal cavity, and rubbed the abdominal wall with fingers to make the liquid flow in the abdominal cavity. Inject the liquid in the sterilized tube, and then lavage once with 10mL of pre-cooled PBS solution, the operation is the same as above. Centrifuge 250g of the combined lavage fluid at 4°C for 10min, discard the supernatant. Add 2mL of erythrocyte lysate to dissolve the erythrocytes, Slightly oscillate twice, 5s each time...
Embodiment 3
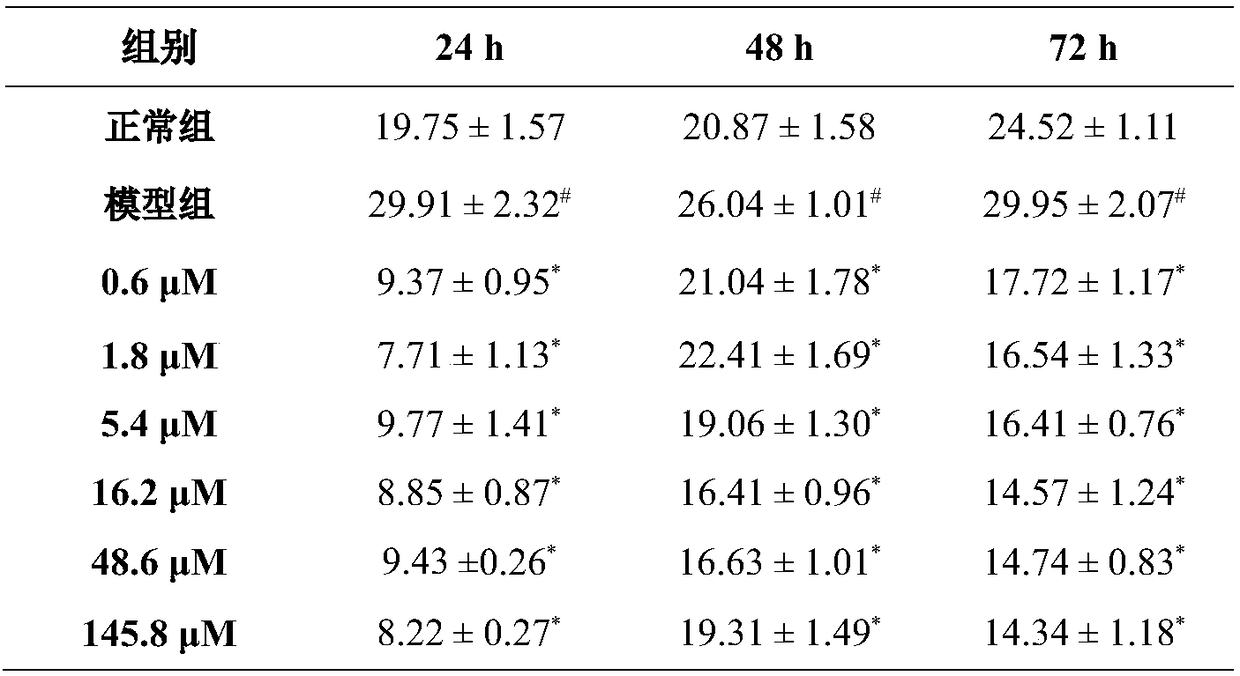
[0050] Example 3. Evaluation of the effect of hydroxysafflower yellow A on the release of TF and PAF from rat abdominal aortic endothelial cells stimulated by LPS
[0051] 1. Experimental materials
[0052] Male clean grade SD rats, 180-220g, LPS, hydroxysafflor yellow A, ECM medium, 24-well plate, tissue factor (TF) ELISA kit, platelet activating factor (PAF) ELISA kit.
[0053] 2. Experimental method
[0054] After killing the rats by cervical dislocation, immerse them in 75% ethanol for 5 minutes, open the chest and abdominal cavity layer by layer, fully expose the thoracic and abdominal aortas, separate the surrounding tissues, and separate the aorta from the proximal end to the iliac The branches of the common artery were put into a petri dish containing PBS, the adipose tissue and fibrous tissue of the adventitia of the blood vessel were aseptically stripped, and the lumen of the blood vessel was washed with PBS. Cut the aorta into small pieces of about 1.5mm×1.5mm, pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com