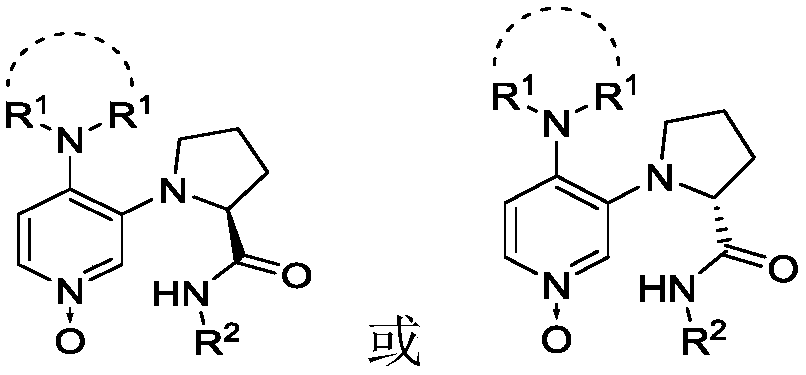
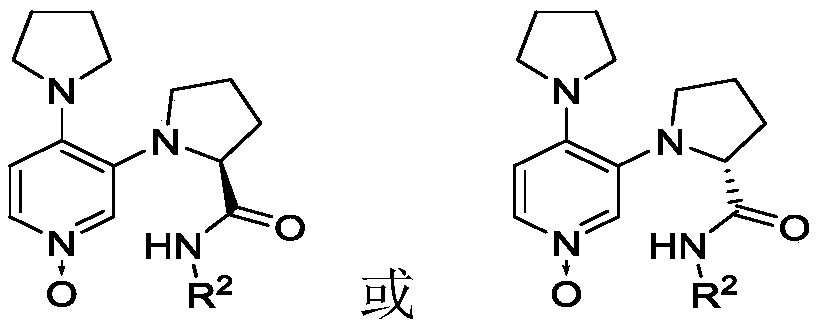
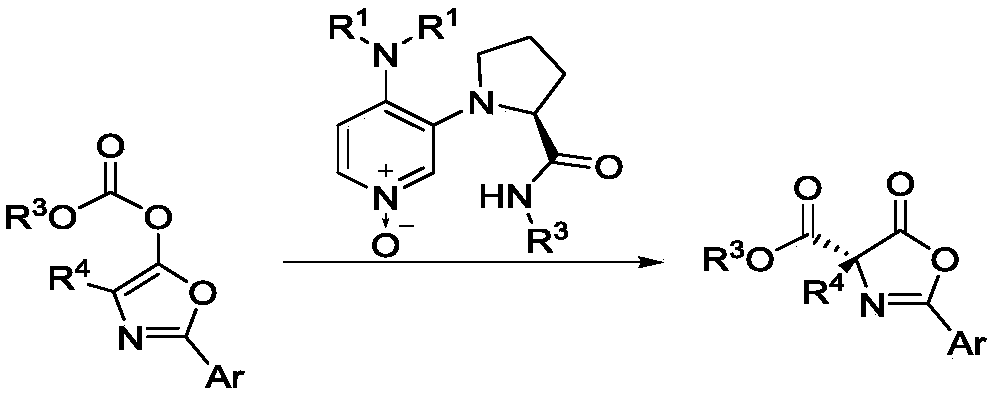
3,4-diaminopyridine oxynitride chiral catalyst and application thereof in Steglich rearrangement
A technology of diaminopyridine nitrogen oxides and chiral catalysts, which is applied in the field of new chiral catalysts and asymmetric reactions, can solve the problems of poor enantioselectivity, low catalyst activity, and high cost. Strong regulation, high catalytic activity, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Chiral 4-Nitro-3-(2-(2,6 Diisopropylbenzamido)pyrrolidinyl)pyridine 1-oxide
[0039]
[0040] In a 100mL flask was added L-2-(2,6 diisopropylbenzamido)pyrrolidine (5.48g, 20mmol), triethylamine (27.7mL, 200mmol, 10eq), 3-bromo-4-nitropyridine Nitrogen oxides (5.45g, 25mmol, 1.25eq) and tetrahydrofuran (35mL) were stirred overnight at 60°C to obtain a crude product which was separated by column chromatography to obtain 7.83g of a bright yellow solid with a yield of 95%, >99% ee. CHIRALCEL IA, n-hexane / 2-propanol=80 / 20, flow rate=1.0mL / min, λ=250nm, retention time: 10.843min.
[0041] 1 H NMR (400MHz, CDCl 3)δ8.21(s,1H),7.79–7.69(m,2H),7.62(s,1H),7.29(d,J=7.6Hz,1H),7.13(d,J=8.0Hz,2H), 4.51(t, J=7.6Hz, 1H), 3.84(td, J=10.4, 5.6Hz, 1H), 3.01(t, J=8.8Hz, 1H), 2.91–2.65(m, 3H), 2.28–2.14 (m,2H),2.08–1.94(m,1H),1.17(d,J=6.8Hz,6H),1.01(s,6H). 13 C NMR (100MHz, CDCl 3 )δ170.1, 146.1, 140.6, 133.6, 130.8, 130.0, 129.8, 128.9, 123.6, 122.7, 64.2, 54.0, 32.0, ...
Embodiment 2
[0046] Synthesis of Chiral 4-Chloro-3-(2-(2,6 Diisopropylbenzamido)pyrrolidinyl)pyridine 1-oxide
[0047]
[0048] Add chiral 4-nitro-3-(2-(2,6 diisopropylbenzamido)pyrrolidinyl)pyridine 1-oxide (2.06g, 5mmol), acetyl chloride (3.57 mL, 50mmol, 10eq), glacial acetic acid (10mL), the temperature was raised to 90-110°C for reaction, after the reaction was detected by TLC, the reaction solution was poured into ice water, and the weak alkalinity was adjusted with sodium hydroxide solution, and the obtained crude product was passed through Separated by column chromatography, 1.4 g of brown solid was obtained, with a yield of 70%, >99% ee. CHIRALCEL IA, n-hexane / 2-propanol=90 / 10, flow rate=1.0mL / min, λ=250nm, retention time: 12.632min(minor), 27.205min(major).
[0049] 1 H NMR (600MHz, CDCl 3 )δ8.09(d,J=2.4Hz,1H),7.84–7.69(m,2H),7.26(d,J=7.2Hz,1H),7.21–7.16(m,1H),7.12(d,J =7.8Hz,2H),4.64–4.58(m,1H),4.11–4.03(m,1H),3.30–3.23(m,1H),2.81(s,2H),2.61–2.52(m,1H), 2.29–2.21(m,1H),2...
Embodiment 3
[0054] Synthesis of Chiral 4-Pyrrolidinyl-3-(2-(2,6 Diisopropylbenzamido)pyrrolidinyl)pyridine 1-oxide
[0055]
[0056] Add chiral 4-chloro-3-(2-(2,6 diisopropylbenzamido)pyrrolidinyl)pyridine nitroxide (200mg, 0.5mmol) and pyrrolidine (1mL) to a 15mL sealed tube at 140 The reaction was carried out at ℃, and the reaction was completed. After vacuum distillation, the crude product was obtained and separated by column chromatography to obtain 133.0 mg of a light brown solid, with a yield of 61%, >99% ee. CHIRALCEL IA, n-hexane / 2-propanol=90 / 10, flow rate=0.5mL / min, λ=250nm, retention time: 20.270min(minor), 35.368min(major).
[0057] 1 H NMR (400MHz, CDCl 3 )δ8.36(s,1H),8.32(s,1H),7.77(dd,J=7.2,2.0Hz,1H),7.22(t,J=7.6Hz,1H),7.08(d,J=7.6 Hz,2H),6.57(d,J=7.2Hz,1H),4.30(t,J=7.2Hz,1H),3.67–3.57(m,1H),3.45–3.34(m,2H),3.25–3.14 (m,2H),2.84–2.71(m,3H),2.50–2.41(m,1H),2.28–2.18(m,1H),2.14–2.04(m,1H),2.03–1.93(m,3H) ,1.91–1.82(m,2H),1.01(d,J=6.8Hz,12H). 13 C NMR (150MHz, CDCl 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com