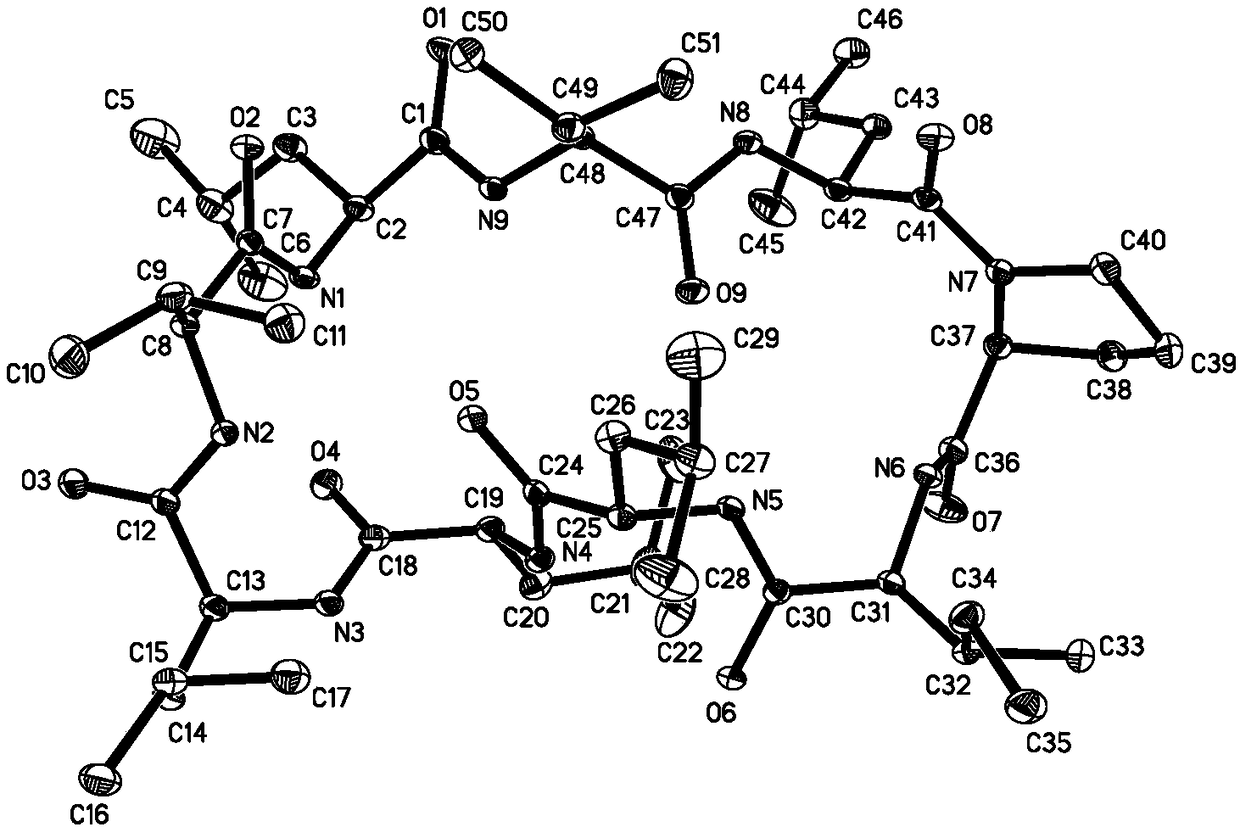
Cyclic peptide GG-8-6 as well as synthesis method and application thereof in preparation of drug for treating HCC (hepatocellular carcinoma)
The technology of a cyclic peptide and compound is applied in the application field of the preparation of anti-cancer drugs, which can solve the problems of short half-life, hindered application, easy enzymatic hydrolysis, etc., and achieve the effects of simple preparation steps, configuration maintenance, and high practical value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1, synthetic cyclic peptide GG-8-6
[0072] 1) Synthetic linear peptide
[0073] (1) Take the resin: take a certain amount of Fmoc-Leu-Wang resin, and swell it in DMF for 30min;
[0074] (2) Removal of the Fmoc protecting group: Stir and react with nitrogen in 20% piperidine / DMF solution for 30 minutes; then wash with equal volume of DMF repeatedly for 3 times, each time for 3 minutes;
[0075] (3) Ninhydrin detection: take a small amount of the reacted resin in a test tube, add 2 drops of 5% (volume fraction, the same below) ninhydrin / ethanol solution, 1 drop of pyridine and 1 drop of 80% phenol ethanol solution, heat About 1 minute; the resin is blue-purple or purple, indicating that the deprotection is successful, and proceed to the next step; if the resin does not change color, it means that the deprotection is not successful, repeat the above reaction step (2) until the resin is blue-purple or purple. ;
[0076] (4) Formation of peptide bonds: Add 3 t...
Embodiment 2
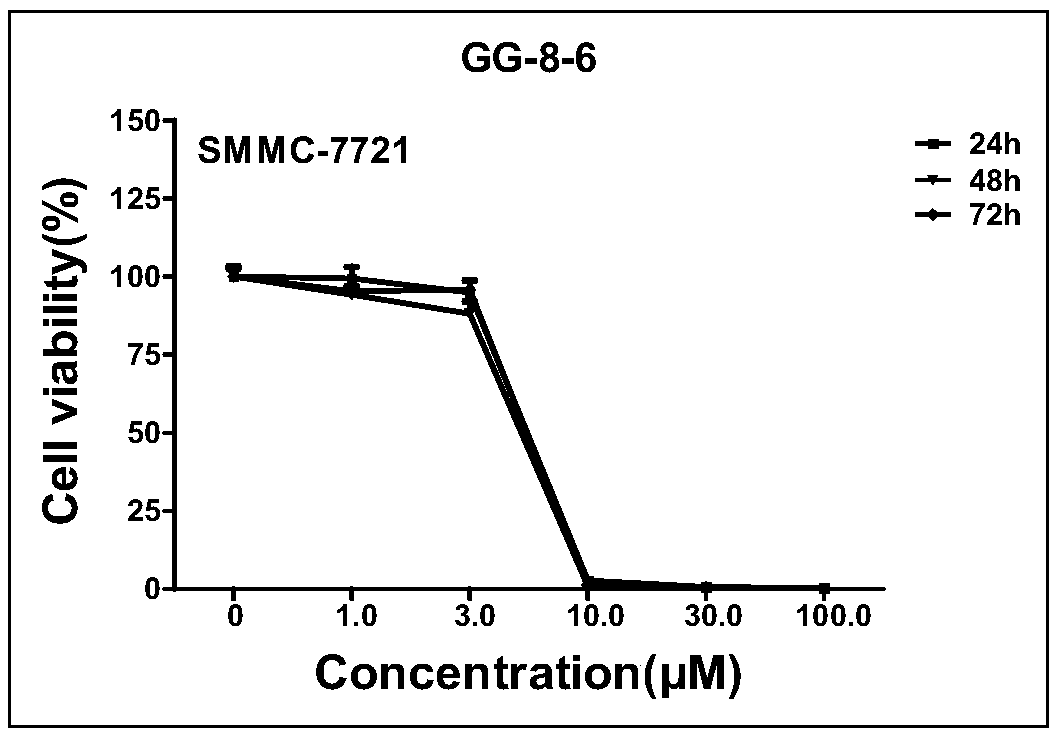
[0093] Embodiment 2, GG-8-6 anti-liver cancer activity experiment in vitro
[0094] The cytotoxicity of the compounds was determined by MTT assay. Cells in the logarithmic growth phase were taken, prepared into a cell suspension with DMEM medium containing 10% fetal bovine serum, and inoculated in a 96-well culture plate, and three replicate wells were set for each concentration of the compound. 100 μl per well (containing 5000 tumor cells), placed at 37°C, 5% CO 2 inside the thermostat. After the compound has acted for a certain period of time, 20 μl of MTT solution was added to each well, and incubated at 37° C. for 4 hours. Afterwards, discard the supernatant, add 150 μL DMSO, use a microplate reader to detect the OD value at 490 nm, and repeat the experimental results three times;
[0095] Cell viability (%)=(OD administration group-OD blank group) / (OD control group-OD blank group)×100%,
[0096] The results showed that the half maximal inhibitory concentration (IC 50...
Embodiment 3
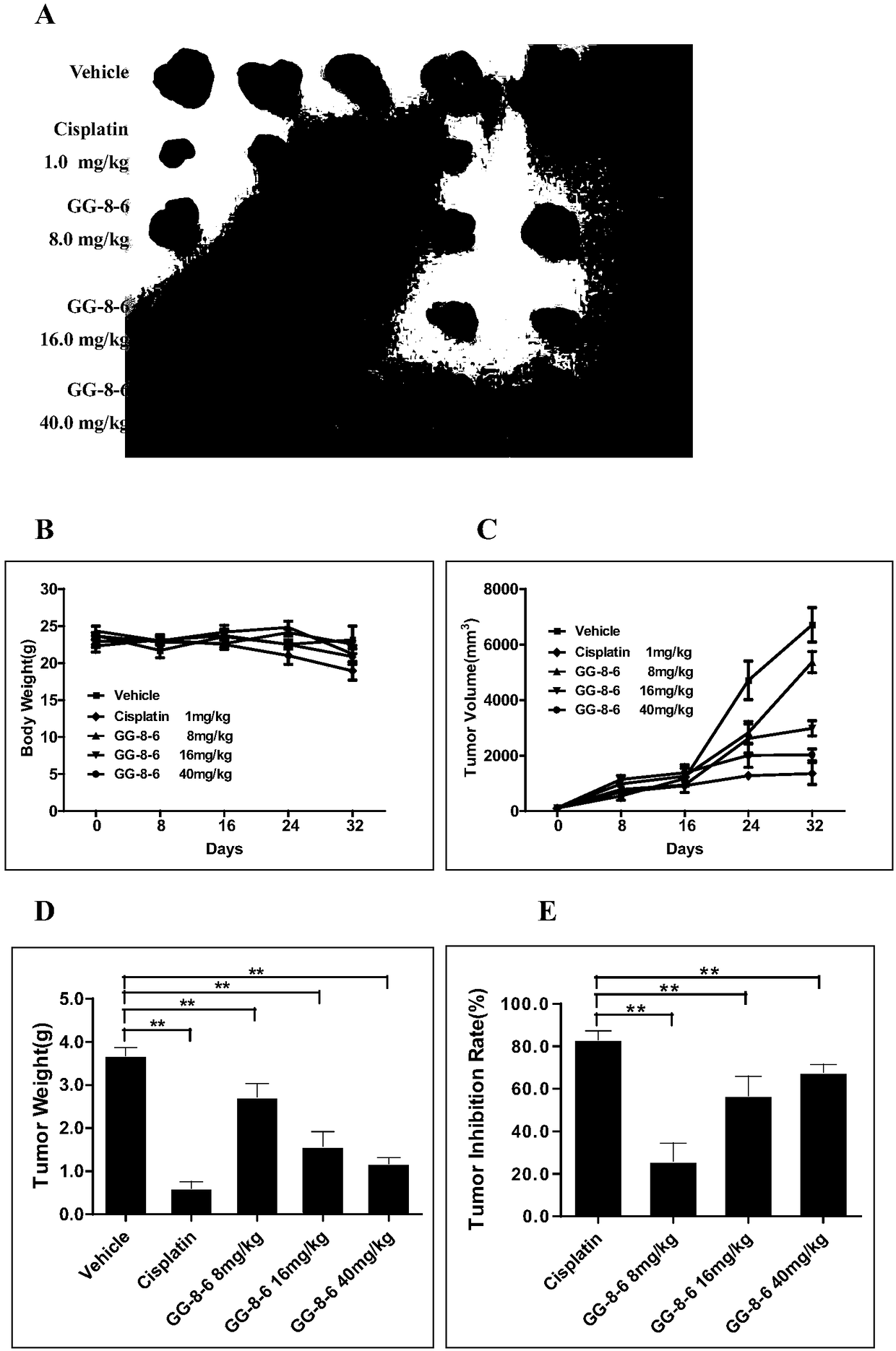
[0097] Example 3, GG-8-6 in vivo anti-liver cancer activity experiment
[0098] Human SMMC-7721 liver cancer cells were cultured in vitro, and the cells in the logarithmic growth phase were taken, and the cell suspension was prepared with serum-free DMEM medium, and inoculated into several nude mice subcutaneously in the forelimbs, and tumors of appropriate size grew after feeding for a period of time After that, the nude mice were sacrificed and the tumors were taken out to prepare the tumor cell suspension. The tumor mass was removed under aseptic conditions, the necrotic tissue was removed, several tumor masses were mixed, cut into small pieces, ground evenly with a glass tissue homogenizer, and then transferred Put it into a sterile culture bottle, add an appropriate amount of serum-free DMEM culture solution to obtain the cell mother solution, count and adjust the cell concentration to 1×10 7 / mL, put the cells on ice; take 50 nude mice, female, 15-18g, introduce them int...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com