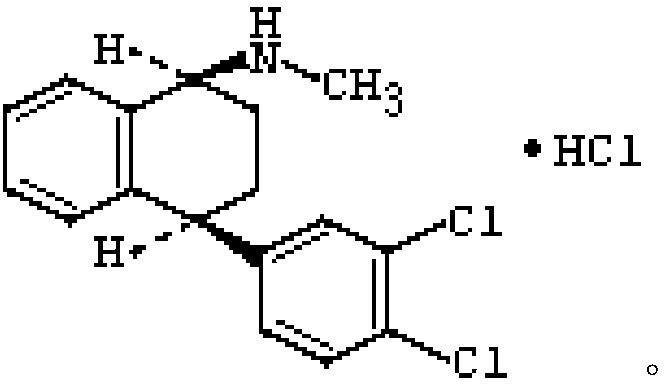
Enteric coated tablet containing sertraline hydrochloride and preparation method thereof
A technology of sertraline hydrochloride and enteric-coated tablets, which is applied in the direction of medical preparations containing active ingredients, medical preparations without active ingredients, and pill delivery, which can solve the problems of low dissolution rate and stability, high requirements for excipients, and technical problems. Complexity and other issues, to achieve the effect of low price of excipients, reduction of adverse reactions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028]
[0029] Its preparation method is
[0030] (1) the sertraline hydrochloride of prescription quantity and the lactose of prescription quantity, dibasic calcium phosphate dihydrate, croscarmellose sodium, hypromellose and magnesium stearate are mixed in mixer for 10min, obtain Intermediates after total blending;
[0031] (2) The intermediate after the total mixing is pressed into tablets according to the weight of the theoretical tablet to obtain plain tablets;
[0032] (3) methacrylic acid copolymer, triethyl citrate, Tween 80 and talcum powder of recipe quantity are dissolved in 80% ethanol, the coating liquid of preparation concentration 8%; And coating liquid is added coating machine Inside;
[0033] (4) Put the sertraline hydrochloride tablets into the coating pan, preheat, and when the tablet bed temperature reaches 40°C, spray the slurry, control the tablet bed temperature to about 40°C, and the coating liquid flow rate to be 40g / min. The surface...
Embodiment 2
[0035]
[0036] Its preparation method is
[0037] (1) microcrystalline cellulose, calcium carbonate, croscarmellose sodium, hypromellose and magnesium stearate of the sertraline hydrochloride of the prescription quantity and the prescription quantity are mixed in a blender for 10min to obtain Intermediates after total blending;
[0038] (2) The intermediate after the total mixing is pressed into tablets according to the weight of the theoretical tablet to obtain plain tablets;
[0039] (3) methacrylic acid copolymer, triethyl citrate, Tween 80 and talcum powder of recipe quantity are dissolved in 80% ethanol, the coating liquid of preparation concentration 8%; And coating liquid is added coating machine Inside;
[0040] (4) Put the sertraline hydrochloride tablets into the coating pan, preheat, and when the tablet bed temperature reaches 40°C, spray the slurry, control the tablet bed temperature to about 40°C, and the coating liquid flow rate to be 40g / min. The surface ...
Embodiment 3
[0042]
[0043] Its preparation method is
[0044] (1) Sertraline hydrochloride of prescription quantity and microcrystalline cellulose of prescription quantity, microcrystalline cellulose, croscarmellose sodium, hypromellose and magnesium stearate are mixed in mixer for 10min , to obtain the intermediate after the total blending;
[0045] (2) The intermediate after the total mixing is pressed into tablets according to the weight of the theoretical tablet to obtain plain tablets;
[0046] (3) methacrylic acid copolymer, triethyl citrate, Tween 80 and talcum powder of recipe quantity are dissolved in 80% ethanol, the coating liquid of preparation concentration 8%; And coating liquid is added coating machine Inside;
[0047] (4) Put the sertraline hydrochloride tablets into the coating pan, preheat, and when the tablet bed temperature reaches 40°C, spray the slurry, control the tablet bed temperature to about 40°C, and the coating liquid flow rate to be 40g / min. The surfac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com