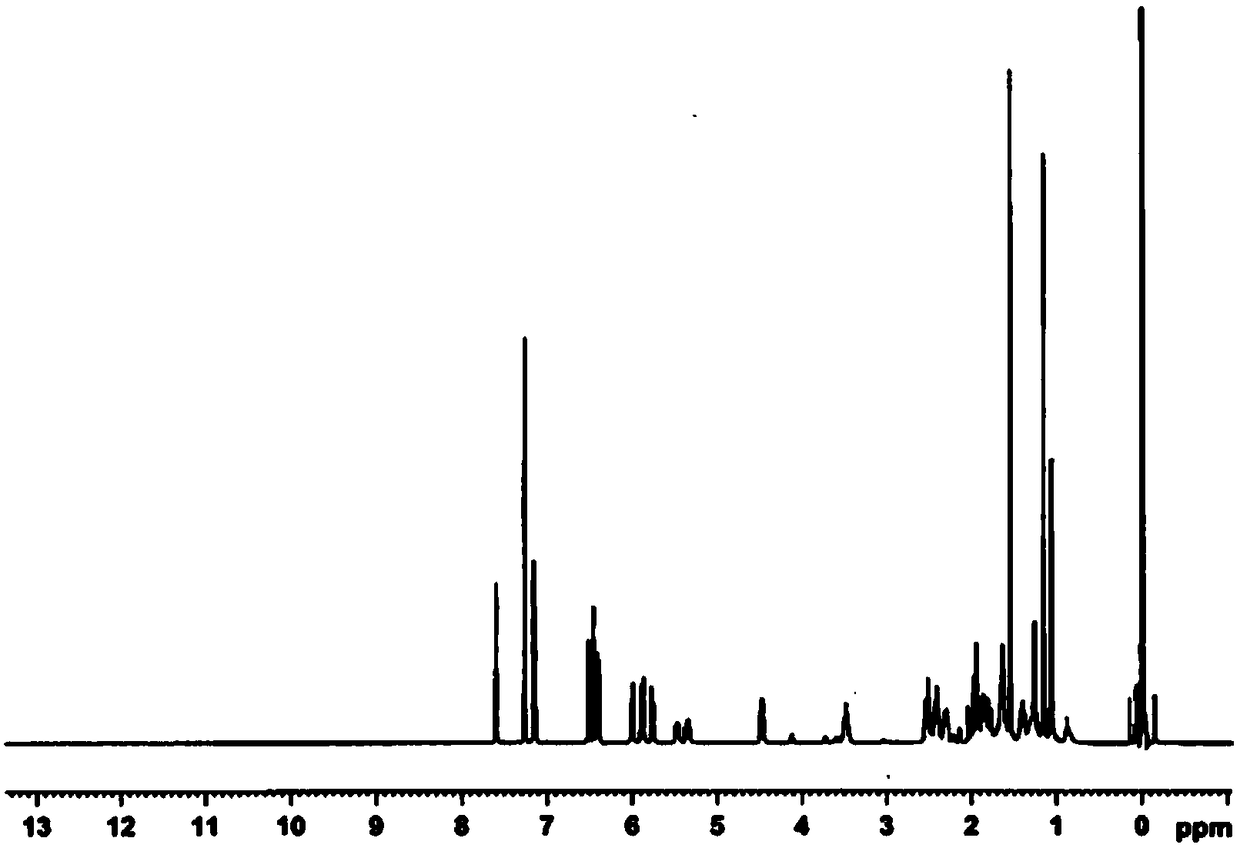
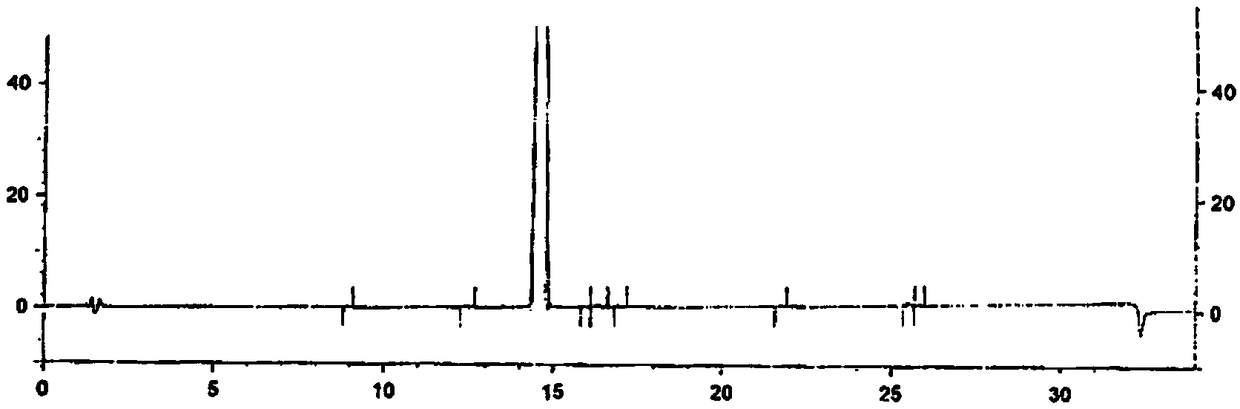
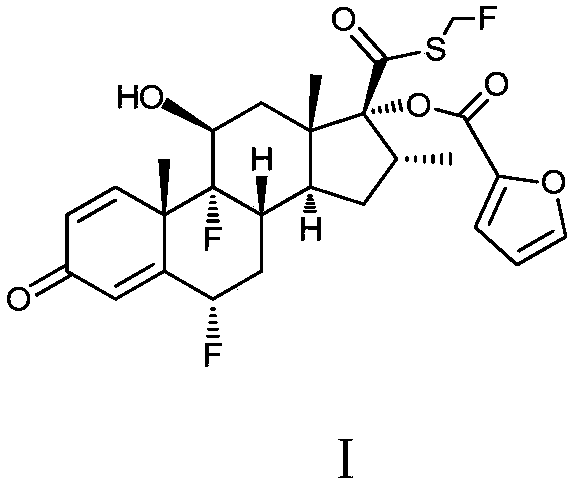
Preparation method of high-purity fluticasone furoate
A fluticasone furoate and high-purity technology, which is applied in the field of preparation of high-purity fluticasone furoate, can solve the problems of complex process, low yield, and low removal efficiency, and achieve the goals of shortening the existence time, high conversion rate, and improving yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preparation of formula III compound:
[0069] 1) Add the compound of formula V (50.0g), butanone (500mL) and diethylamine (38.29g) into the reaction flask at a temperature of ≦30°C; then add furoyl chloride (32.74 g), react for 0.5-1h until the reaction is complete (HPLC detection, calculated by the peak area according to the normalization method, if the compound of formula V in the raw material is ≦1.0%, the reaction can be considered complete). Then add a mixed solution of diethanolamine (19.89g) and water (250mL) and react for 1-1.5h; then add water (250mL), and adjust the pH value to 2-3 with 10% hydrochloric acid (72g). ℃ temperature, stirring for 30min, a large amount of solid precipitated. Filter the solid, rinse with water (100mL); add water (250mL) and acetone (250mL) to the solid, stir for 30min at a temperature of 20-30°C, filter, rinse with water (100mL), and then dissolve the solid at a temperature of 70-80°C After drying, 61.80 g of the compound of f...
Embodiment 2
[0072] 1) Add the compound of formula III (50.0g) prepared in Example 1 and methanol (250mL) into the reaction flask at a temperature ≦30°C under nitrogen protection, then add potassium carbonate (35.88g), and heat up to 25-35°C , reacted for 16h until the reaction was complete (HPLC detection, calculated by the normalization method with the peak area, the compound of formula III≦1.0% in the raw material, the reaction can be considered complete), and a mixture containing the compound of formula II was prepared.
[0073] 2) Add purified water (375mL), methanol (125mL), ethyl acetate (125mL) and toluene (125mL) to the mixture containing the compound of formula II prepared in the previous step, stir for 10min, and stand to separate the phases. Add ethyl acetate (125mL) and toluene (125mL) to the water phase, stir for 10min, and let stand to separate the phases; the water phase is then cooled to 10-20°C, and the pH value is adjusted to 2- 3; add methyl ethyl ketone (750mL) and sod...
Embodiment 3
[0077] With reference to the preparation method of Example 2, respectively adopt the conditions listed in the following table to prepare the compound of formula I (except the conditions listed in the table below, other conditions are the same as in Example 2)
[0078]
[0079] Using the conditions in experimental group a, the refined product of the compound of formula I was finally obtained, with a yield of 81.12%, a purity of 99.43%, and total impurities of 0.57%.
[0080] Using the conditions in the experimental group b, the refined product of the compound of formula I was finally obtained, with a yield of 85.50%, a purity of 98.75%, and total impurities of 1.27%.
[0081] Using the conditions in experimental group c, more solids were precipitated and phase separation was impossible, which made it difficult to carry out the subsequent steps.
[0082] Using the conditions in the experimental group d, the refined product of the compound of formula I was finally obtained, wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com