Method applying AlphaLISA to detect dengue virus and corresponding combination and kit
A dengue virus and detection method technology, applied in the field of biomedicine, can solve the problems of long time consumption, difficulty in adapting to large sample detection, cumbersome operation steps, etc., and achieve good specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of AlphaLISA kit for detecting dengue virus.
[0032] The buffers of the capture antibody and the detection antibody, which are paired antibodies, were exchanged to desalt and purify and remove sodium azide (NaN 3 ). Zeba from Thermo Fisher Scientific TM Put the centrifugal desalting column into the corresponding collection tube, and centrifuge at 1500g for 1min to remove the storage solution. Wash 2 more times with 1×PBS. Add the antibody stock solution and put on a new collection tube, centrifuge at 1500g for 1min, keep the antibody solution in the collection tube, and prepare the antibody concentration at 1mg / ml.
[0033] Detection antibody conjugated to biotin. Add the purified 1mg / mL detection antibody with pH ≥ 7.0 to 2mg / mL NHS-ChromaLink-biotin biotin labeling reagent solution and adjust the volume to 200uL, incubate at 23°C for 2 hours, and then use Zeba TM The biotin-labeled antibody was collected after desalting spin column purific...
Embodiment 2
[0038] Example 2: Evaluation test of the AlphaLISA kit for detecting dengue virus.
[0039] Detection reagents: Prepare relevant reagents according to the method described in Example 1.
[0040] Detection steps: add the sample to be tested or positive quality control or negative quality control to a 384-well microplate, then add receptor beads coated with capture antibody, then add biotin-labeled detection antibody, and incubate at 23°C After 30-60 minutes, add the donor microbeads coated with streptavidin, incubate for another 20-30 minutes in a dark environment, and then detect the fluorescence value.
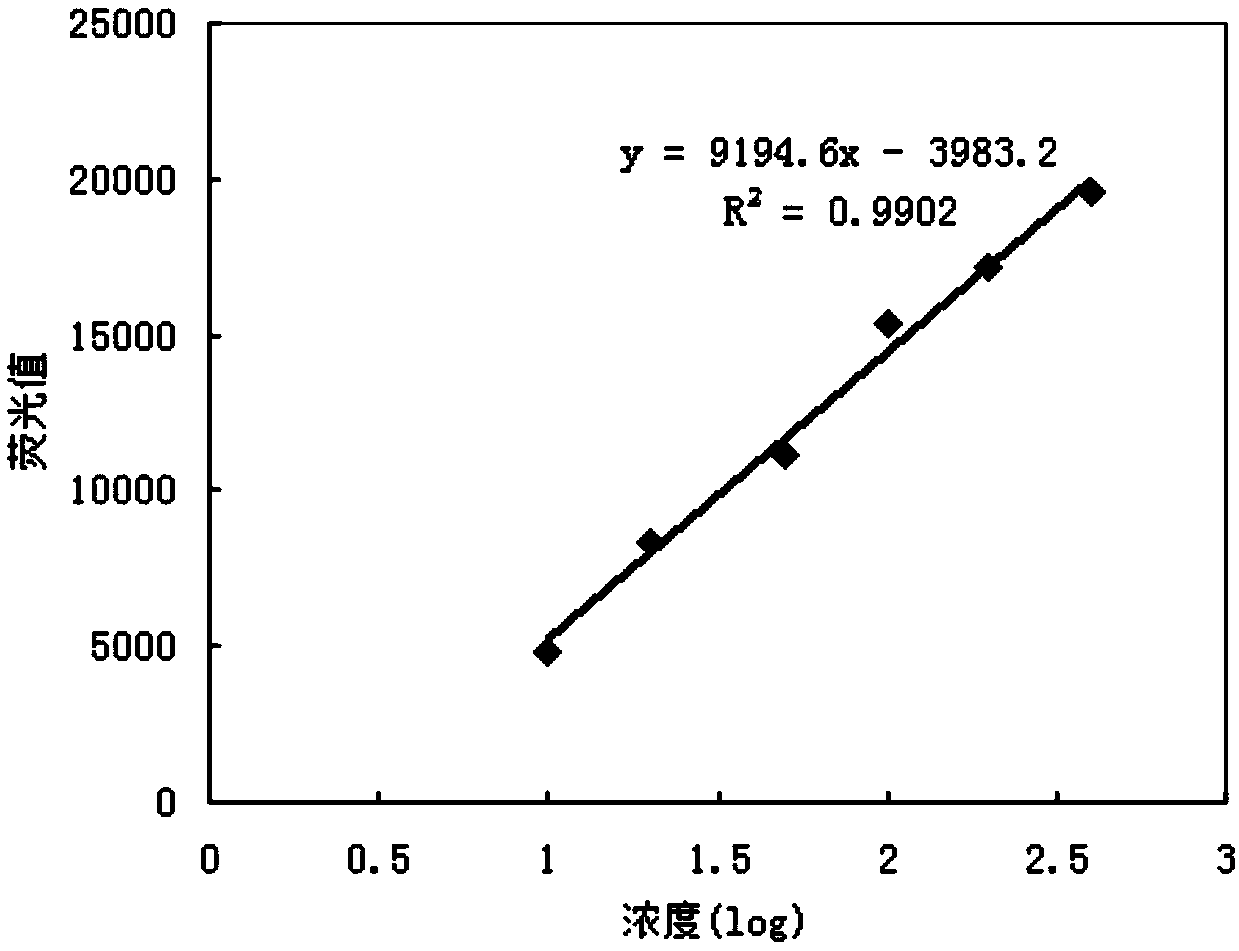
[0041] 1. Linear correlation analysis
[0042] The positive quality control product and the negative quality control product of gradient dilution are detected simultaneously with the method of the present invention, take the logarithm of the sample concentration as the abscissa, the fluorescent signal value is the ordinate, and fit a linear curve to obtain dengue virus NS1 ...
Embodiment 3
[0049] Embodiment 3: performance evaluation test
[0050] Method 1 is the AlphaLISA method established by the present invention. The second method is a commercial kit (dengue fever NS1 rapid detection reagent from cortez company in the United States), and its detection sensitivity is 6 ng / mL.
[0051] Sample: Randomly select 100 clinical serum samples, including dengue positive cases and normal people, and use the above two methods for detection at the same time.
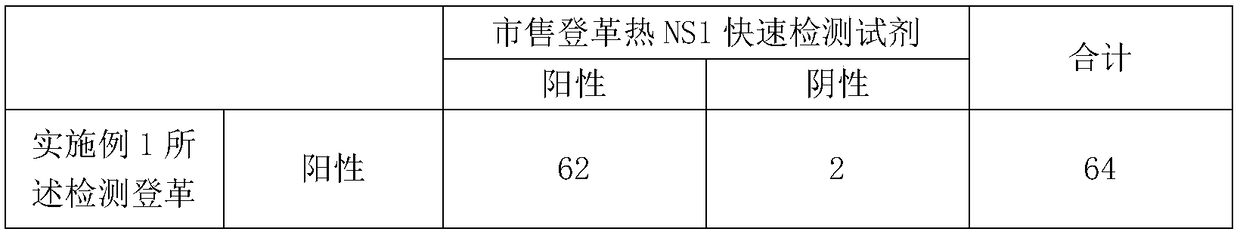
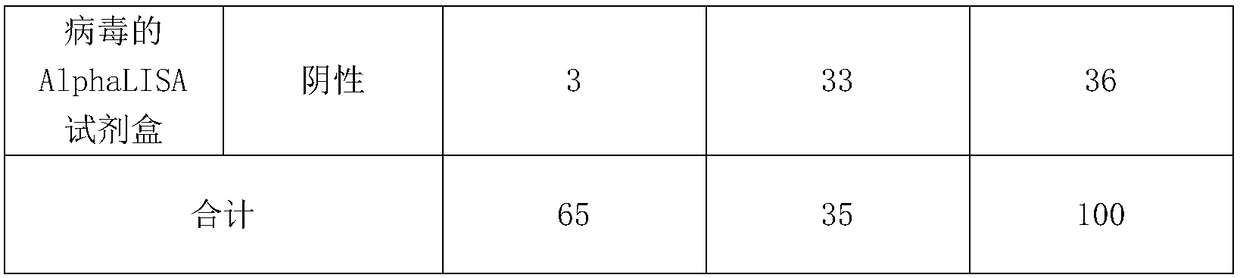
[0052] After the test was completed, the negative-positive coincidence rate and the percentage of consistency were analyzed. The results are shown in Table 1.
[0053] Table 1 Qualitative detection methodological performance evaluation results
[0054]
[0055]
[0056] According to the test results, the positive coincidence rate is 95.38%, the negative coincidence rate is 94.29%, and the degree of agreement is 95%.
[0057] Conclusion: The AlphaLISA kit for detecting dengue virus provided by the present in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com