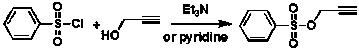Green synthesis method of prop-2-yn-1-yl benzenesulfonate
A green synthesis, propargyl ester technology, applied in the preparation of pesticide intermediates, the pharmaceutical field, can solve the problems of easy polymerization and volatilization, high reactivity, difficult to improve product purity, etc., to achieve mild reaction conditions, high yield, operation Simple and convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
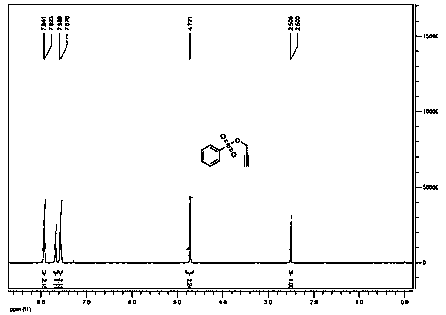
[0026] Add 5 kg of benzenesulfonyl chloride, 1.9 kg of propargyl alcohol and 5 kg of water into a 50-liter reactor, turn on the cooling device, control the temperature at 15°C, and add 3 kg of sodium carbonate / 10 kg of aqueous solution dropwise within 2 hours; Stir the reaction at 15°C, the reaction of benzenesulfonyl chloride in the gas phase detection system is complete, static liquid separation, the organic phase is washed with 2 × 5 kg of salt water, dried over anhydrous sodium sulfate, and distilled under reduced pressure to collect 142-148°C / 4mmHg fractions, 3.8 kg of propargyl benzenesulfonate was obtained with a yield of 69% and a gas phase purity of 99.1%.
Embodiment 2
[0028] Add 5 kg of potassium carbonate and 20 kg of water into a 50-liter reactor, stir to dissolve and add 1.9 kg of propargyl alcohol, turn on the cooling device, control the temperature at 10°C, and add 5 kg of benzenesulfonyl chloride dropwise within 2 hours; the dropwise addition is completed , continue to stir the reaction at 10°C, the reaction of benzenesulfonyl chloride in the gas phase detection system is complete, static separation, the organic phase is washed with 2 × 5 kg of salt water, dried over anhydrous sodium sulfate, distilled under reduced pressure, and collected at 142-148°C / 4mmHg Distillate, obtain 4.2 kilograms of propargyl benzenesulfonates, yield 76%, gas phase purity 99.4%.
Embodiment 3
[0030] Add 5 kg of benzenesulfonyl chloride, 2 kg of propargyl alcohol and 10 kg of water into a 50-liter reactor, turn on the cooling device, control the temperature at -5°C, and add 12 kg of 10% sodium hydroxide aqueous solution dropwise within 2 hours; Continue to stir the reaction at -5°C, the reaction of benzenesulfonyl chloride in the gas phase detection system is complete, static separation, the organic phase is washed with 2 × 5 kg of salt water, dried over anhydrous sodium sulfate, distilled under reduced pressure, and collected at 142-148°C / 4mmHg Distillate, obtain 4.8 kilograms of propargyl benzenesulfonates, yield 87%, gas phase purity 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com