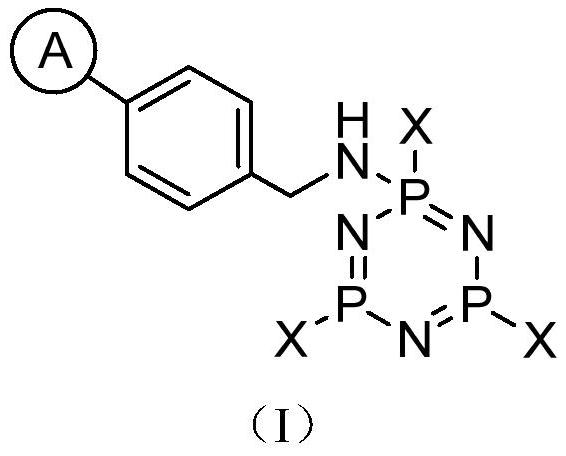
Cyclic oligomeric phosphazene base catalyst loaded on polystyrene microspheres and its preparation method and use
A technology of polystyrene microspheres and polystyrene, applied in the field of organic chemistry, can solve the problems of limited catalyst types, complex synthesis methods, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
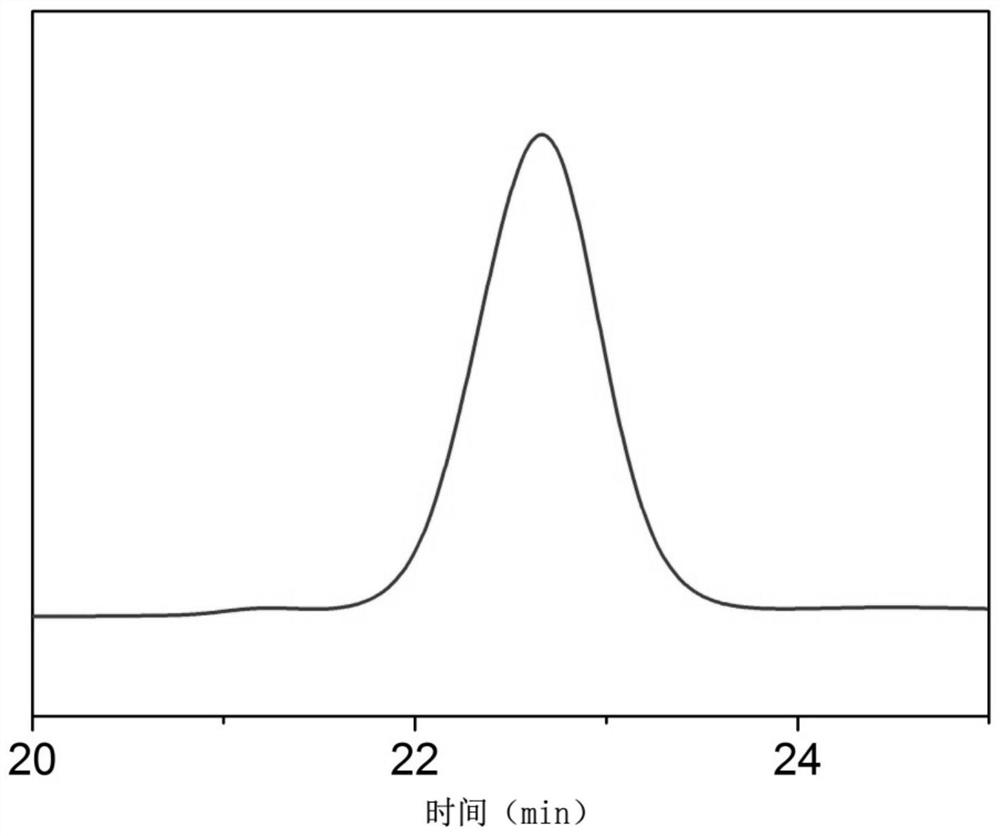
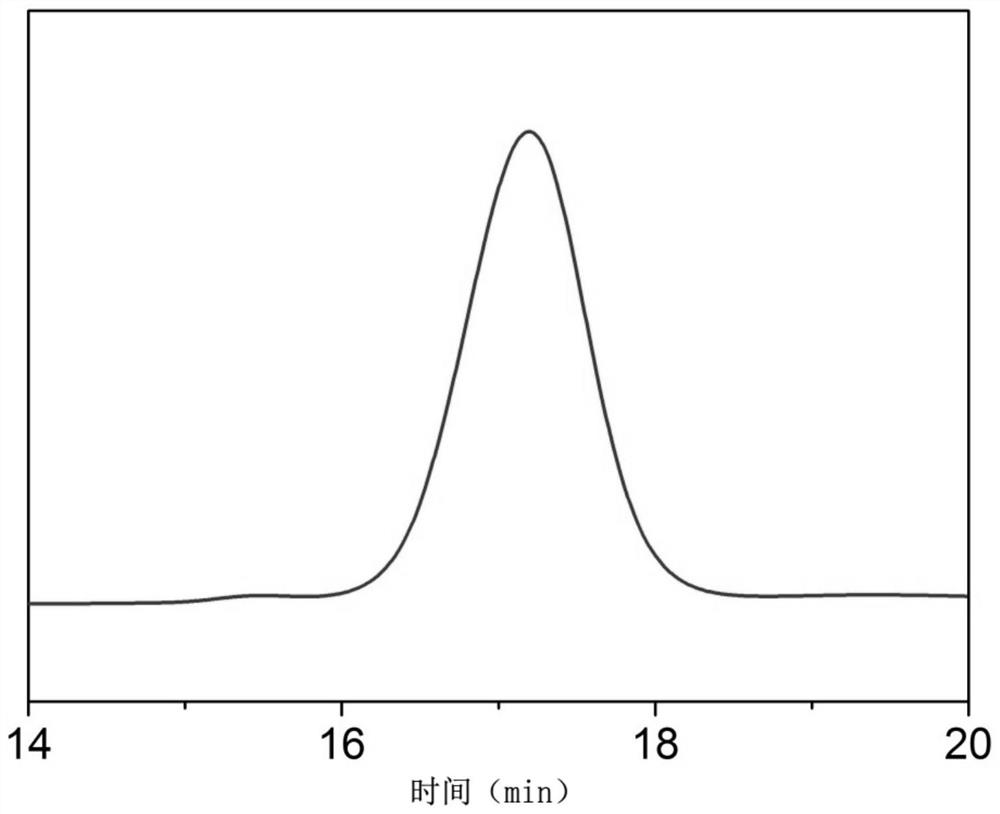
Image
Examples
preparation example Construction
[0088] Preparation method of catalyst
[0089] In a second aspect of the invention, the present invention has proposed a method of preparing a catalyst of the above examples. According to an embodiment of the invention, the method comprises:
[0090] (1) The chloromethyl substituted polystyrene-divinylbenzene is a vacuum drying treatment. Specifically, the chloromethyl substituted polystyrene-divinylbenzene, which may be sufficiently washed with methanol before use, and then substituted with methanol-washed chloromethal-substituted polystyrene-divinyl group The beads of the beads were dried in vacuo at 50 ° C for 2 to 24 h.
[0091]According to an embodiment of the present invention, the chloromethyl substituted polystyrene-divinylbenzene-difinylbenzene can be 100 to 600 mesh, preferably 200 to 400 mesh.
[0092] According to an embodiment of the invention, the functional group density of the chloromethyl substituted polystyrene-divinylbenzene-di-diobenzene bead may be 1 to 4 mmol...
Embodiment 1
[0138] First step, preparation of aminocyreopyrene microspheres
[0139] Using methanol thorns 11 g of methyl chloromethyl substituted polystyrene-divinylbenzene multi-p-copolymer bead (1% DVB, 200 ~ 400 mesh, 1 to 1.3 mmol cl / g), 50 ° C, vacuum drying 24 hours . Under a nitrogen atmosphere, anhydrous tetrahydrofuran (THF) was added to allow the copolymer beads for 24 hours, separated from the solid, dried in vacuo at 50 ° C for 24 hours.
[0140] 10 g of pre-treated chloromethyl polystyrene microspheres were placed in 150 ml N, N-dimethylformamide (DMF), added (3.4 g, 17.8 mmol) of phthalimide potassium, at 50 Stir at ° C for 24 hours. After the reaction, the tan polymer bead was filtered. DMF (20 mL × 3), methanol (20 ml × 3), and methanol (20 mL × 5) were washed again. The resulting polymer was dried in vacuo at 50 ° C for 24 hours, weighed 11.8 g.
[0141] The above-mentioned brown polymer 11.8 g was added to anhydrous ethanol, 2 ml of hydrated compound, and refluxed for 24 ...
Embodiment 2
[0148] First step, the preparation of aminocyreolystyrene microspheres is the same as in Example 1
[0149] Step 2, three [three (diethylamine) phosphorus nitrocene] Preparation of trichloropathic acid
[0150] Phalochloride (20.9 g, 0.1 mol) was added to a three-mouth flask equipped with mechanical stirring, and 75 ml of anhydrous methylene chloride was added under a nitrogen protection, which was placed in a low-temperature bath of -40 ° C to maintain vigorous stirring. Dietamine (62 mL, 0.6 mol) was added dropwise to the reaction system, and the temperature of the solution was lower than -30 ° C. After passing, the temperature is naturally raised to 20 ° C and the reaction is continued for 2 hours. The system temperature was again reduced to 0 ° C, and the ammonia gas was accented to saturation, raised to 20 ° C, and the ammonia gas was continued for 3 hours until the stirring surface did not precipitate. The solvent was filtered off, and the solvent was evaporated under reduce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com