Lipase mutant with improved heat stability as well as preparation method and application thereof
A technology of thermal stability and mutant, applied in the field of molecular biology, can solve the problems of poor thermal stability, poor stability, low catalytic ability, etc., and achieve the effect of improving thermal stability and thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Selection of hotspot amino acids
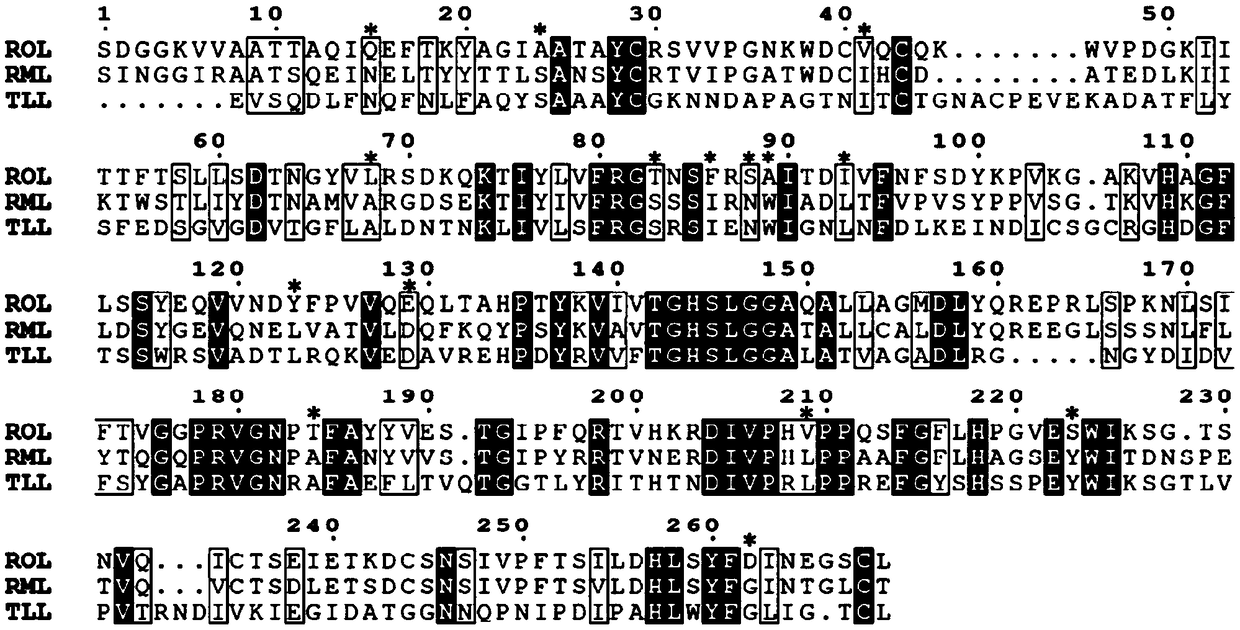
[0052] Multiple sequence alignment was performed using the online analysis website ClustalW (http: / / www.genome.jp / tools-bin / clustalw). Lipase RML from Rhizomucor miehei and lipase TLL from Thermomyces lanuginosa have better heat resistance than lipase ROL from Rhizopus oryzae, And it has high homology with ROL, the sequence homology is 53.4% and 29.7%, respectively. Therefore, these two lipases were selected for multiple sequence alignment with ROL to identify potential sites that could enhance the thermostability of ROL.
[0053] Each sequence was uploaded in FASTA format and the results were uploaded to Espript 3.0 (http: / / espript.ibcp.fr / ESPript / cgi-bin / ESPript.cgi) and plotted for a more intuitive alignment.
[0054] The result is as figure 1 As shown, amino acid residues marked with asterisks indicate potential sites selected based on multiple sequence alignment results that may affect the thermal stability of ROL. Among ...
Embodiment 2
[0074] 1. Design of disulfide bonds
[0075]Upload the crystal structure of ROL (PDB ID: 1LGY) to Disulfide by Design 2 (DbD2, http: / / cptweb.cpt.wayne.edu / DbD2 / index.php), and perform calculations. After the initial analysis of the protein structure, the prediction of the protein structure begins, analyzing the potential disulfide bond positions. The results showed that 32 pairs of amino acid residues may form disulfide bonds after mutation to cysteine (Table 2). Score and classify predictions based on energy, bad contacts, thermal mobility, sequence separation, etc. According to the scoring results, the 9 pairs of amino acids with the highest score (>90) were selected for mutation.
[0076] Table 2 Disulfide bond design results
[0077]
[0078]
[0079] Cysteine was introduced at the specific site of lipase ROL by whole-plasmid PCR. PCR primers are shown in Table 3.
[0080] Table 3 Mutation primer design table based on disulfide bond design results
[0081] ...
Embodiment 3
[0092] Enhanced thermostability through combinatorial mutations of hot spots and disulfide bonds
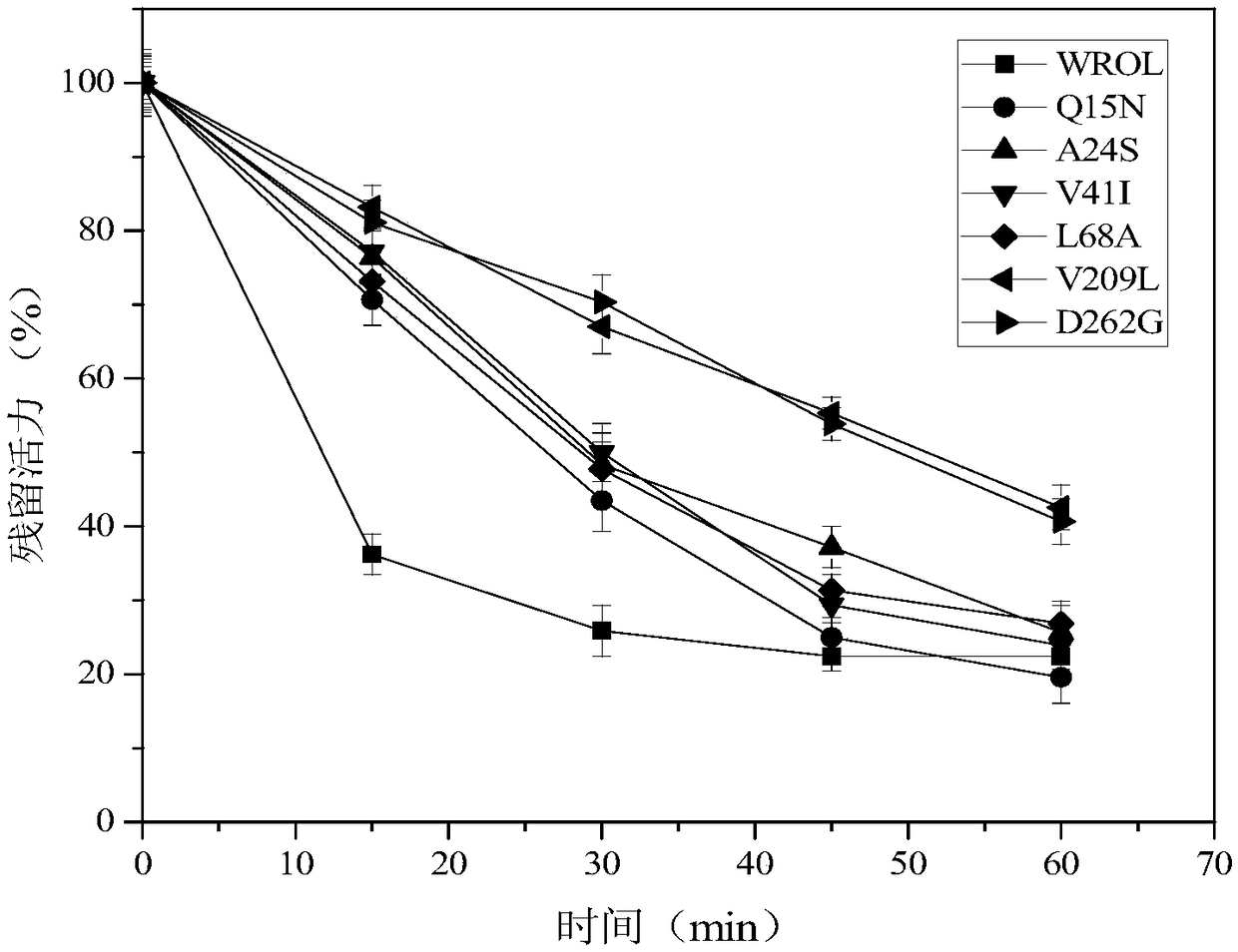
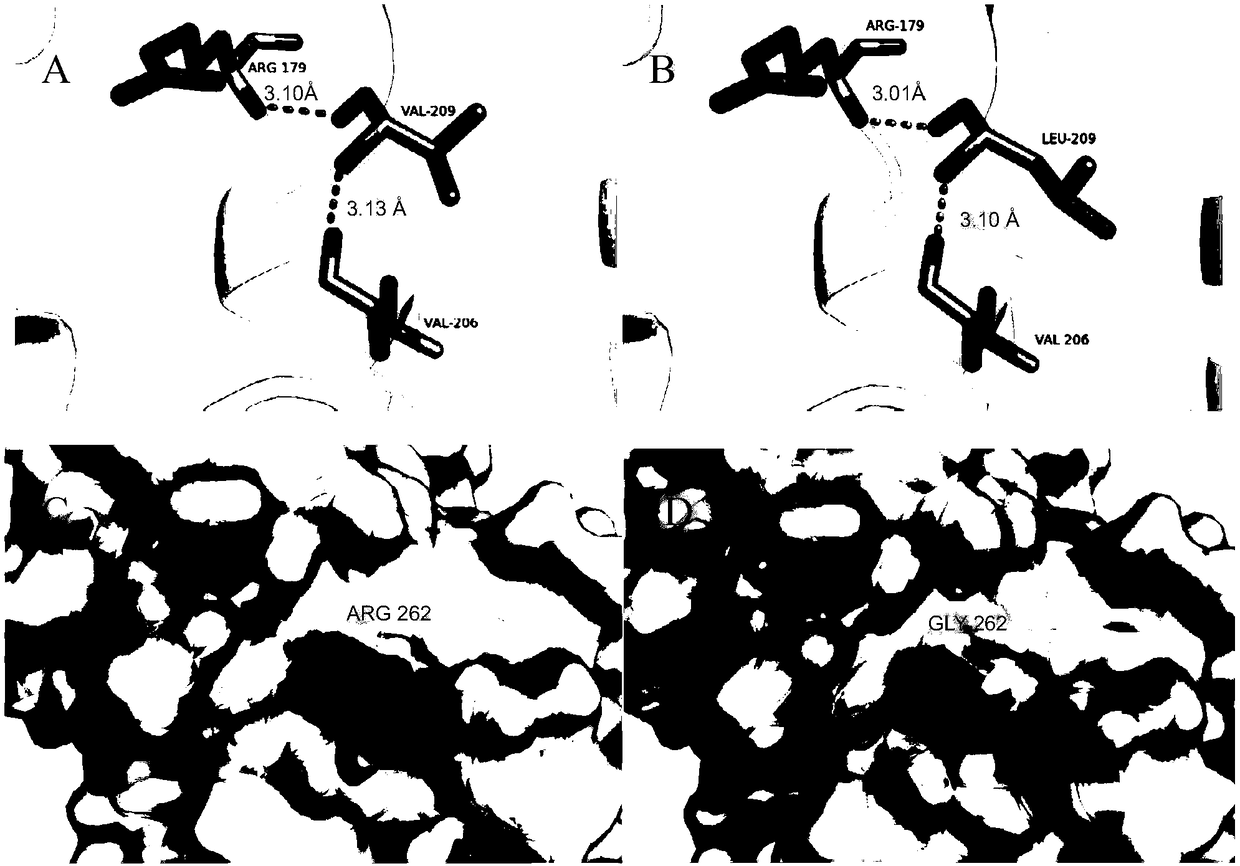
[0093] It is possible to obtain mutants with further improved thermostability by combining mutations at the amino acid sites that affect the thermostability of the enzyme. Based on the results obtained above, we performed combined mutations on ROL, and the mutation sites were V209L, D262G and E190C and E238C. Mutant V209L-D262G still retains about 76% of its initial activity after being incubated at 55°C for 30 minutes;
[0094] Since the mutant E190C / E238C showed improved thermostability at 65°C, the thermostability test of the combined mutations was also performed at 65°C. Such as Figure 7 As shown, compared with the mutant E190C / E238C, all the combined mutants showed enhanced thermal stability, among which, the triple-point mutant V209L-E190C-E238C still retained about 42% of the initial activity; D262G-E190C-E238C retained about 45% of the initial activity after incubatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com