Bacterial quorum sensing inhibitors as well as preparation method and application thereof
A technology of isomers and medicinal salts, applied in the field of bacterial quorum sensing inhibitors and its preparation, can solve the problems of reducing the antibacterial efficiency of antibiotics and increasing drug-resistant strains, achieving good economy, simple preparation method, and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Synthetic purification and structure identification of embodiment 1 pyrrolidone derivative General method:
[0100] Take 5.0g of levulinic acid in a three-necked flask, add 10ml of petroleum ether, 2ml of 40% HBr, stir in an ice bath, and protect with nitrogen; take another 10ml of bromine in 20ml of petroleum ether, drop by drop with a normal pressure separatory funnel In the three-necked bottle (about 2 drops / second) drip completely in 35 minutes, and rinse with 20ml of petroleum ether. Heated to 50°C for about 30 minutes, condensed to reflux. After reacting for one hour, a large amount of white solids precipitated. After cooling, they were washed with 10 ml of water, 10 ml of sodium thiosulfate solution (0.5 mol / L), and 10 ml of saturated brine, dried, and rotary evaporated to obtain a crude product. Take about 11.5 g of the obtained crude product, add 75 ml of 98% concentrated sulfuric acid, heat to 120° C., condense and reflux. After reacting for 20 minutes, cool...
Embodiment 2
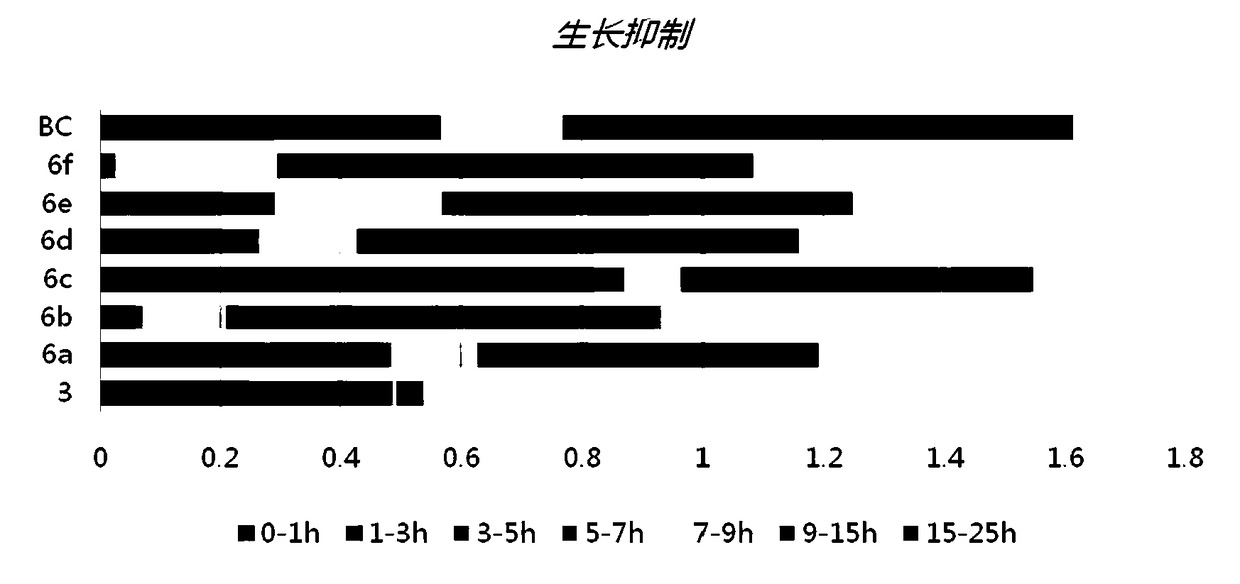
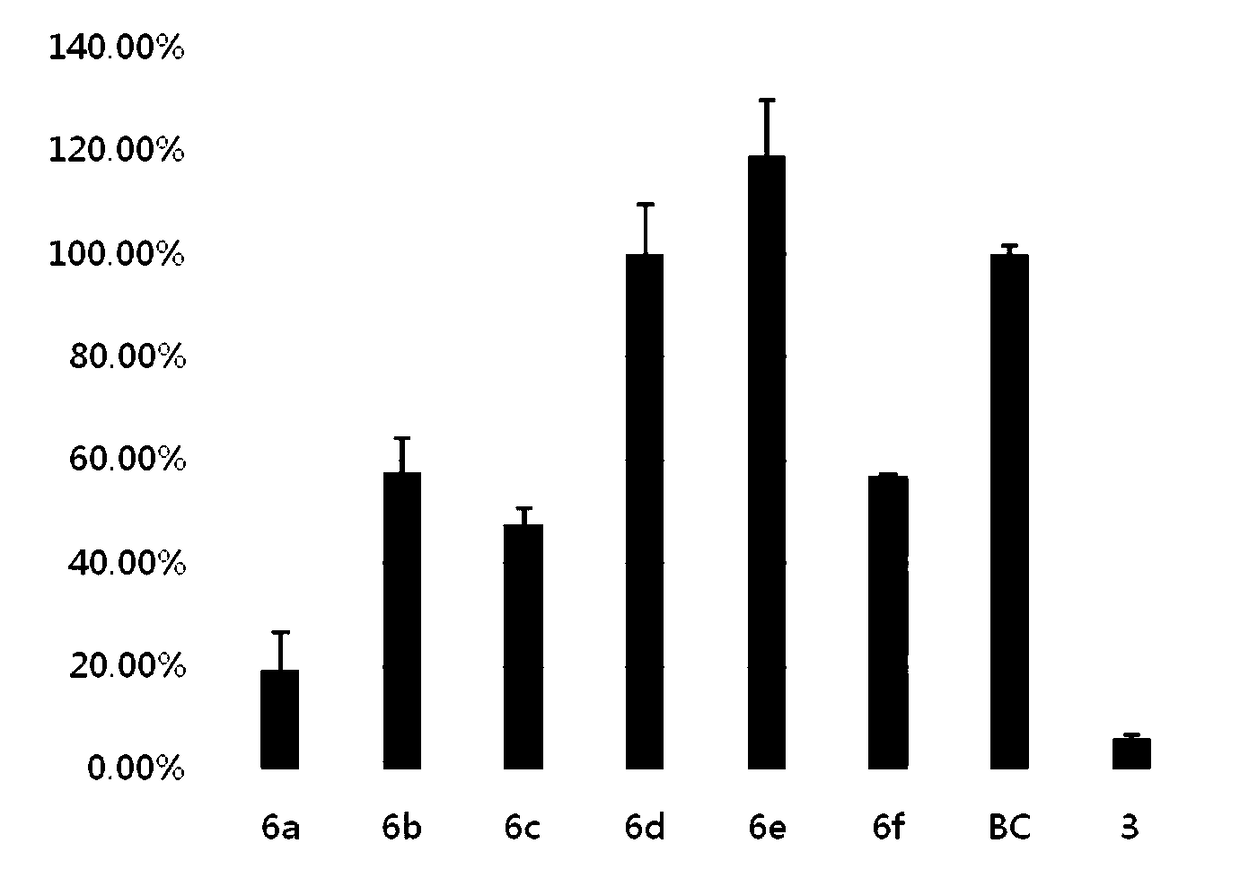
[0109] Example 2 The effect of pyrrolidone derivatives on inhibiting the growth of Pseudomonas aeruginosa PAO1 and the output of pyocyanin:
[0110]Experimental method: Pseudomonas aeruginosa (CGMCC 1.12483, PAO1) was inoculated into LB medium, cultured overnight at 37° C. to obtain Pseudomonas aeruginosa PAO1 bacterial liquid. 3 mL of bacterial liquid was dispensed into test tubes, and 3 μM of each pyrrolidone derivative was added. After culturing for 1, 3, 5, 7, 9, 15, and 25 hours, the absorbance at 620 nm was measured respectively. It can be seen that these compounds have no inhibitory effect on the growth and reproduction of Pseudomonas aeruginosa (3 of which are positive control drugs).
[0111] After that, the original bacterial solution was diluted to OD 620 =0.05, continue culturing for three hours to the logarithmic growth phase. Dilute again to OD 620 = 0.05. The culture solution was dispensed into test tubes by 5 mL. Add 5 μM of each pyrrolidone derivative co...
Embodiment 3
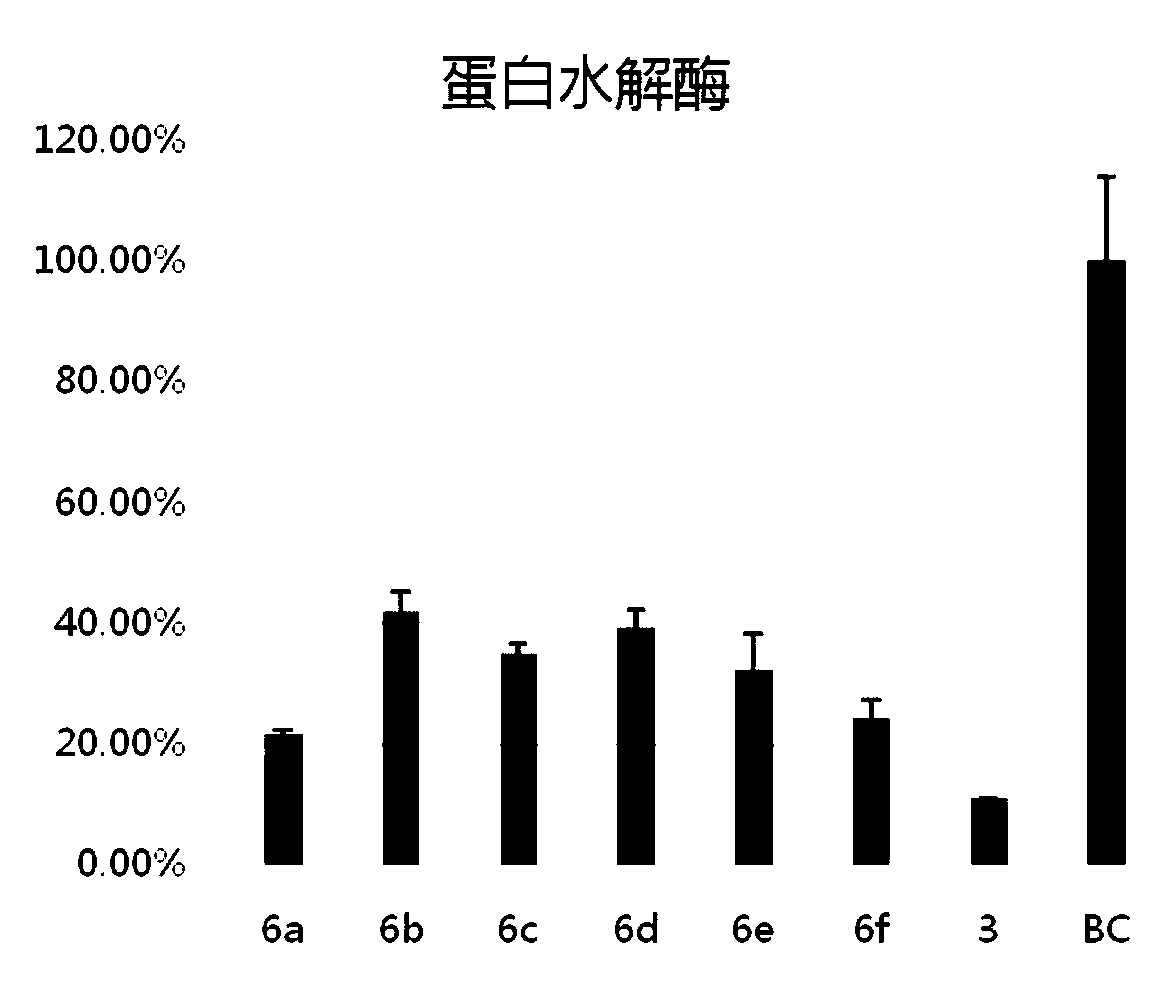
[0113] Example 3 Inhibition of pyrrolidone derivatives to Pseudomonas aeruginosa PAO1 proteolytic enzyme:
[0114] Inoculate Pseudomonas aeruginosa into LB medium and culture overnight at 37°C to obtain Pseudomonas aeruginosa PAO1 bacterial liquid. The bacterial solution was dispensed into 5 mL test tubes, and after adding 5 μM of each pyrrolidone derivative, cultured in a 37° C. incubator for 8 hours. Afterwards, filter 100 μL of the culture solution, dispense it into EP tubes, add 5 mg of azocasein substrate, 1 mL of Tris buffer (10 mM), CaCl 2 (1 mM). The OD440 and OD620 values of the solution were measured after the culture solution was quenched and centrifuged. The expression level of proteolytic enzymes is represented by OD440 / OD620, and the inhibition rate is calculated by comparing the results of the experimental group with the blank control. image 3 It can be seen that these compounds have different degrees of inhibition (58.2%-78.5%) on PAO1 proteolytic enzyme....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com