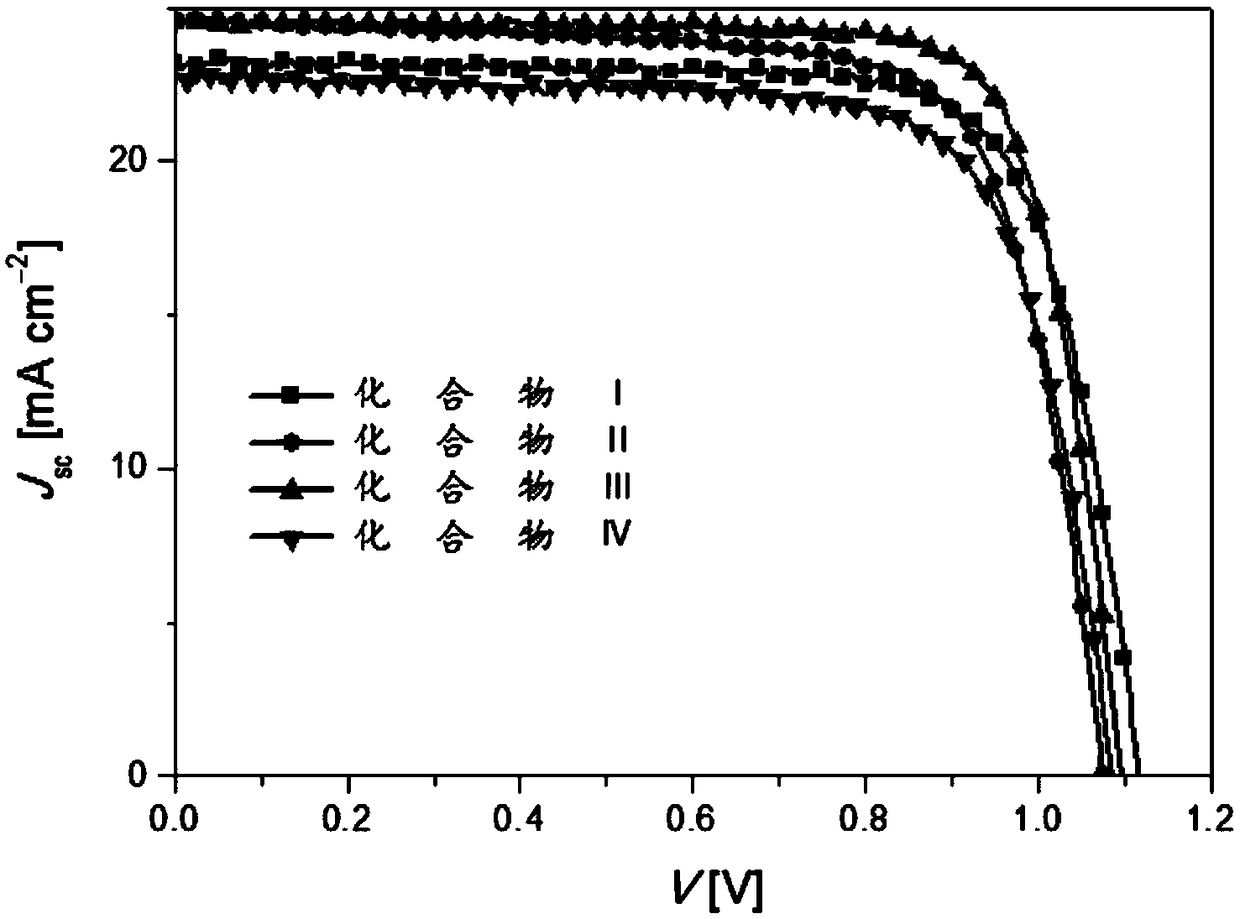
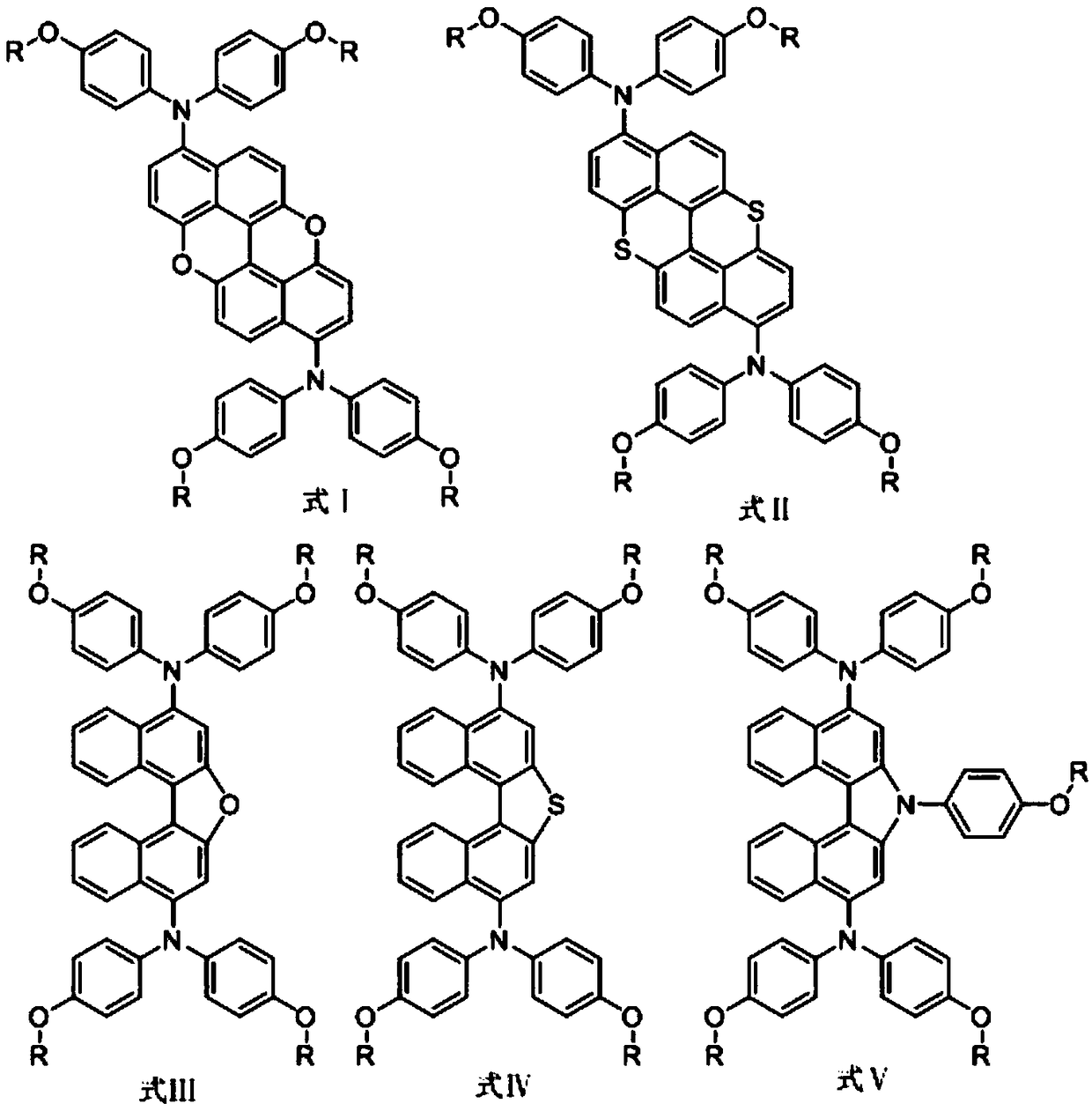
Dinaphtho heterocyclic ring small molecular hole-transport material as well as synthesis method and application thereof
A technology of hole transport materials and heterocyclic small molecules, applied in luminescent materials, chemical instruments and methods, photovoltaic power generation, etc., can solve the problem of low energy conversion efficiency of perovskite solar cells, unstable battery device performance, and expensive materials. and other problems, to achieve the effects of low synthesis cost, improved environmental stability, and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of compound I (the synthetic route is shown in the summary of the invention):
[0051] Synthesis of Intermediate I: Dioxaanthanthrene (2.86g, 10mmol) was dissolved in 2L of dichloromethane to obtain a saturated solution; liquid bromine (3.2g, 20mmol) was slowly added dropwise at -78°C . The resulting mixed solution was slowly raised to room temperature during 5 hours with stirring, and stirred and reacted at room temperature for 2 hours. As the reaction progressed, a yellow solid precipitated out of the reaction system. The resulting solid was filtered by suction and washed to obtain intermediate I (4.36 g, 99% yield).
[0052] Synthesis of Compound Ⅰ: Intermediate Ⅰ (880mg, 2mmol) was mixed with dimethoxydiphenylamine (1.08g, 4.4mmol), tris(dibenzylideneacetone)dipalladium (91mg, 0.1mmol), tetrafluoroborate tris tert-butylphosphine (58mg, 0.2mmol) and sodium tert-butoxide (577mg, 6mmol) were added to 50mL of toluene; under the protection of nitrogen, hea...
Embodiment 2
[0054] Preparation of compound II (the synthetic route is shown in the summary of the invention):
[0055] Synthesis of Intermediate II: Dithioxaanthanthrene (3.14g, 10mmol) was dissolved in 3L of dichloromethane to obtain a saturated solution; liquid bromine (3.2g, 20mmol) was slowly added dropwise at -78°C . The resulting mixed solution was slowly raised to room temperature during 5 hours with stirring, and stirred and reacted at room temperature for 2 hours. As the reaction progressed, a red solid precipitated out of the reaction system. The resulting solid was filtered by suction and washed to obtain intermediate II (4.63 g, 98% yield).
[0056] Synthesis of Compound Ⅱ: Intermediate Ⅱ (944mg, 2mmol) was mixed with dimethoxydiphenylamine (1.08g, 4.4mmol), tris(dibenzylideneacetone) dipalladium (91mg, 0.1mmol), tetrafluoroborate tris Tert-butylphosphine (58mg, 0.2mmol) and sodium tert-butoxide (577mg, 6mmol) were added together into 50mL of toluene, under the protection o...
Embodiment 3
[0058] Preparation of compound III (the synthetic route is shown in the summary of the invention):
[0059] Synthesis of Intermediate III: Dinaphthofuran (2.68g, 10mmol) was dissolved in 100mL of dichloromethane to obtain a saturated solution; liquid bromine (3.2g, 20mmol) was slowly added dropwise at -78°C. The resulting mixed solution was slowly raised to room temperature during 5 hours with stirring, and stirred and reacted at room temperature for 2 hours. As the reaction progressed, a white solid precipitated out of the reaction system. The resulting solid was filtered by suction and washed to obtain intermediate III (4.18 g, 99% yield).
[0060] Synthesis of compound Ⅲ: Intermediate Ⅲ (846mg, 2mmol) was mixed with dimethoxydiphenylamine (1.08g, 4.4mmol), tris(dibenzylideneacetone) dipalladium (91mg, 0.1mmol), tetrafluoroborate tris Tert-butylphosphine (58mg, 0.2mmol) and sodium tert-butoxide (577mg, 6mmol) were added together into 50mL of toluene, under the protection o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com