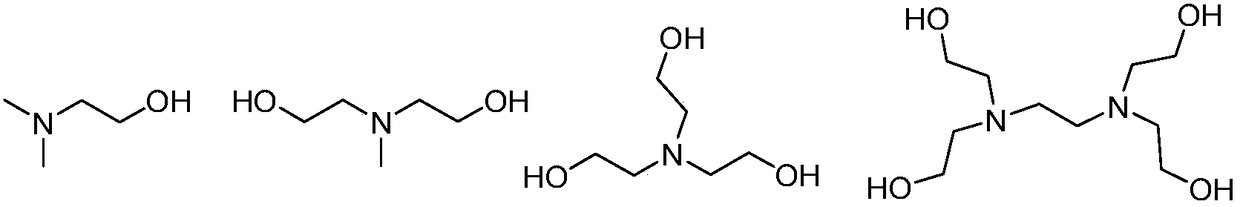
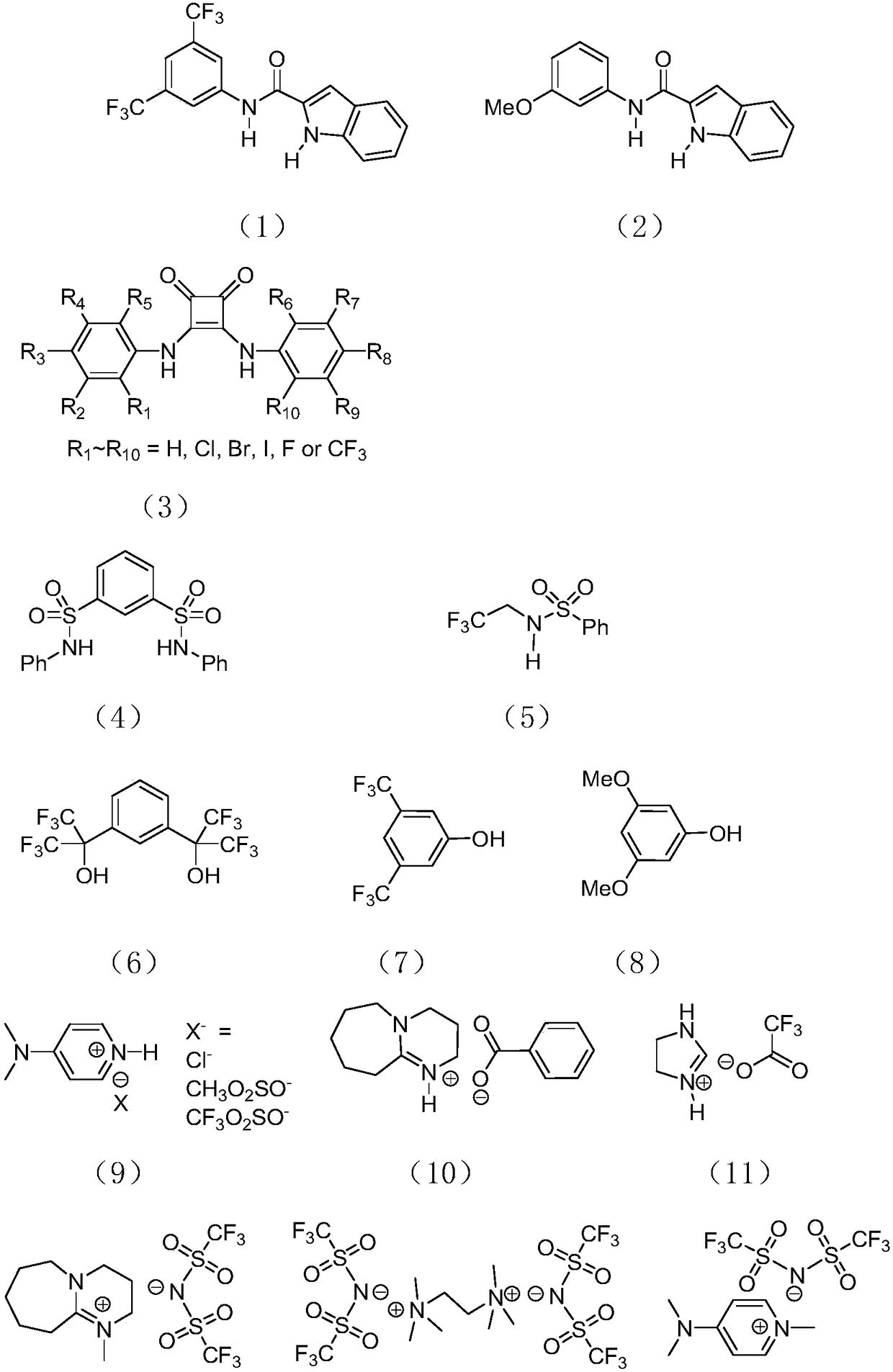
Catalyst for preparing polypeptide through ring-opening polymerization of amino acid N-carboxy anhydride, and method for preparing polypeptide by catalyst
An intracyclic acid anhydride and ring-opening polymerization technology is applied in the field of polypeptide catalysis, which can solve the problems of poor molecular weight controllability, low initiation efficiency of metal complexes, low nucleophilicity of primary amine hydrochloride, etc. Application value, beneficial to separation and purification, the effect of fast polymerization reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Catalyst component A has the structure of formula I, the above catalyst component B has the structure of formula (1), and the acid anhydride monomer in the amino acid ring is γ-benzyl-L-glutamic acid ring acid anhydride monomer. At room temperature, add 37.99 μmol of catalyst component A, 379.87 μmol of catalyst component B and 4ml of organic solvent in a 25mL polymerization bottle after anhydrous treatment, after stirring for 5min, add 1.90mmol of an acid anhydride monomer in the amino acid ring, and then After the polymerization reaction at room temperature for 6 minutes, add an ethanol solution of hydrochloric acid with a volume fraction of 10% to the polymerization bottle to terminate the reaction, pour the reaction solution into ethanol for sedimentation, filter to obtain a white solid, place the gained white solid in a drying oven, and After drying at 40° C. for 24 h, a polypeptide with a net weight of 0.41 g was obtained.
[0036] The conversion rate of α-amino a...
Embodiment 2
[0038] The catalyst component A has the structure of formula I, the catalyst component B has the structure of formula (3), and the amino acid anhydride monomer is N(ε)-benzyloxycarbonyl-L-lysine anhydride monomer. At room temperature, add 32.65 μmol of catalyst component A, 326.46 μmol of catalyst component B and 4 ml of organic solvent into a 25 mL polymerization bottle after anhydrous treatment, after stirring for 5 min, add 1.63 mmol of amino acid anhydride monomer in the ring, and then After the polymerization reaction at room temperature for 6 minutes, add an ethanol solution of hydrochloric acid with a volume fraction of 10% to the polymerization bottle to terminate the reaction, pour the reaction solution into ethanol for sedimentation, filter to obtain a white solid, place the gained white solid in a drying oven, and After drying at 40° C. for 24 h, a polypeptide with a net weight of 0.42 g was obtained.
[0039] The conversion rate of α-amino acid-N-carboxy anhydride ...
Embodiment 3
[0041] Catalyst component A has the structure of formula I, the catalyst component B has the structure of formula (4), and the above-mentioned amino acid anhydride monomer is γ-benzyl-L-glutamic acid anhydride monomer. At room temperature, add 3.80 μmol of catalyst component A, 18.99 μmol of catalyst component B and 4 ml of organic solvent into a 25 mL polymerization bottle after anhydrous treatment. After stirring for 5 minutes, add 1.90 mmol of amino acid anhydride monomer in the ring, and then After the polymerization reaction at room temperature for 30 minutes, add a volume fraction of 10% ethanol solution of hydrochloric acid to the polymerization bottle to terminate the reaction, pour the reaction solution into ethanol for sedimentation, filter to obtain a white solid, place the gained white solid in a drying oven, and After drying at 40° C. for 24 h, a polypeptide with a net weight of 0.41 g was obtained.
[0042] The conversion rate of α-amino acid-N-carboxy anhydride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com