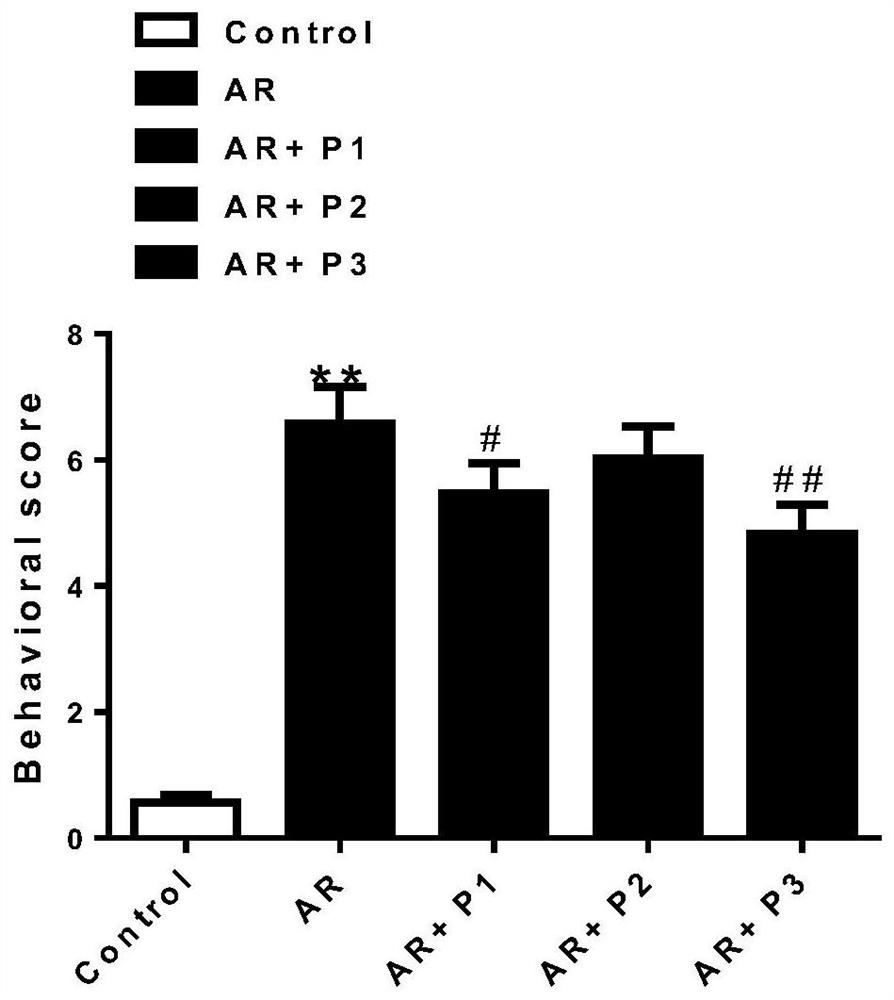
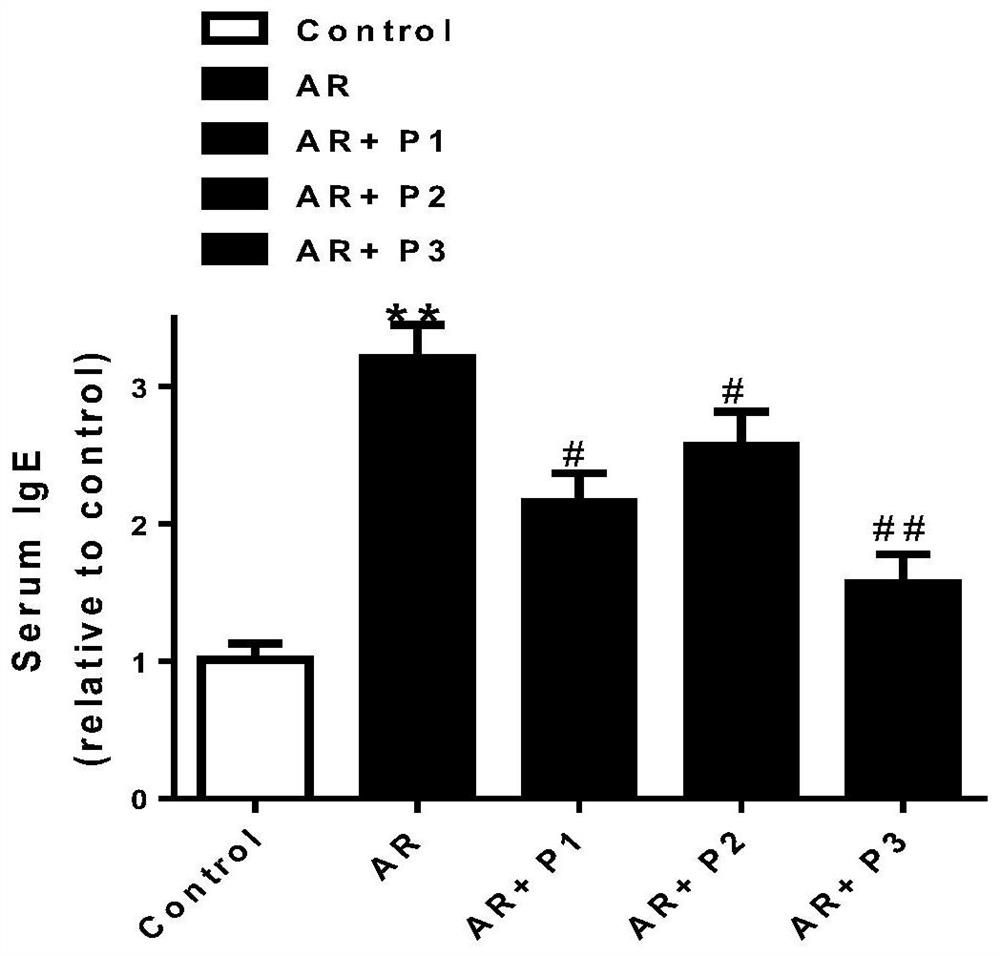
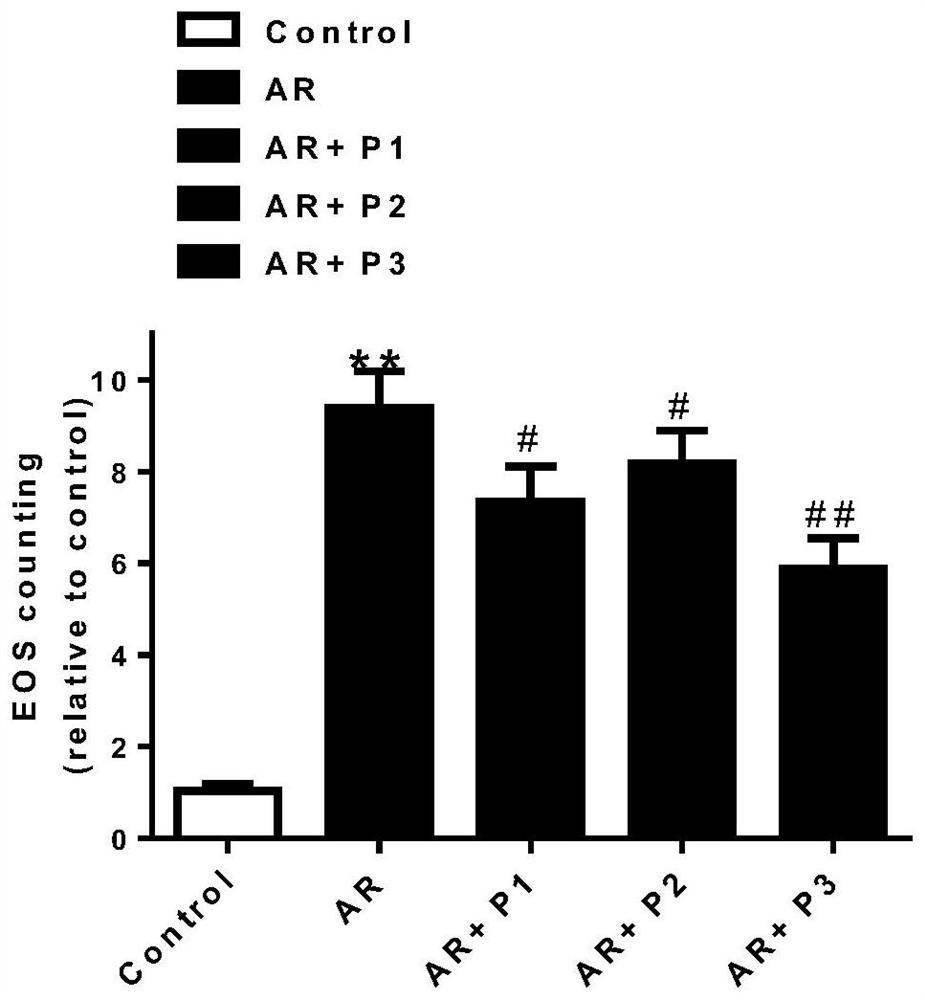
Probiotic composition for preventing and treating allergic rhinitis, nasal preparation and preparation method based on it
A technology for allergic rhinitis and probiotics, used in allergic diseases, drug combinations, medical preparations with inactive ingredients, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] In terms of mass fraction, it includes: 75% medical gel, 8% isomaltooligosaccharide, 1% Sanguobao, and 100% glycerin; The order of magnitude of bacteria is 10 8 ).
[0109] Preparation method:
[0110] (1) Weigh the sterilized medical gel, isomaltooligosaccharide and Sanguobao according to the above ratio, stir at 75°C for 15min, 600-800rpm;
[0111] (2) Inoculate the probiotic powder into the sterilized glycerin, stir at 37°C for 10min, 600rpm;
[0112] (3) After cooling the gel mixture prepared in (1) to 37°C, add the probiotic glycerol mixture prepared in (2), and stir at 37°C for 10 minutes at 600 rpm to obtain the product.
[0113] Note that the Sanguobao used in the embodiment of the present invention is a product that can be purchased on iHerb, and its UPCCode: 797734507287. (The following examples are the same)
Embodiment 2
[0115] In terms of mass fraction, it includes: 75% medical gel, 8% isomaltooligosaccharide, 1% Sanguobao, 100% glycerin; probiotic powder: selected from Lactobacillus acidophilus and Bifidobacterium (acidophilus The mass ratio of Lactobacillus: Bifidobacteria is 3:1, and the order of probiotics contained in each milliliter gel is 10 8 ).
[0116] Preparation method:
[0117] (1) Weigh the sterilized medical gel, isomaltooligosaccharide and Sanguobao according to the above ratio, stir at 75°C for 15min, 600-800rpm;
[0118] (2) Inoculate the probiotic powder into the sterilized glycerin, stir at 37°C for 10min, 600rpm;
[0119] (3) After cooling the gel mixture prepared in (1) to 37°C, add the probiotic glycerol mixture prepared in (2), and stir at 37°C for 10 minutes at 600 rpm to obtain the product.
Embodiment 3
[0121] In terms of mass fraction, it includes: water-soluble chitosan 2%, isomaltooligosaccharide 10%, Sanguobao 1%, NaCl 1%, double distilled water to 100%; probiotic powder: selected from Lactobacillus acidophilus (The order of magnitude of probiotics contained in every milliliter lotion is 10 8 ).
[0122] Preparation method:
[0123] (1) Weigh the sterilized water-soluble chitosan, isomaltooligosaccharide, Sanguobao and NaCl according to the above ratio, stir at 75°C for 15min, 600-800rpm;
[0124] (2) Inoculate the probiotic powder into (1) cooled to 37°C, stir at 37°C for 10min, 600rpm;
[0125] (3) Adjust the pH of (2) to 5.5. If the pH is low, NaOH can be added, and if the pH is high, lactic acid can be added.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com