Method for industrially preparing phenethyl resorcinol based on H2SO4-SiO2 solid acid catalyst
A technology of phenethyl resorcinol and solid acid catalyst, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of oxidative discoloration, corrosion of stainless steel equipment, limited application, etc., and achieve the reaction time Short, avoid strong corrosive effect, low cost of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
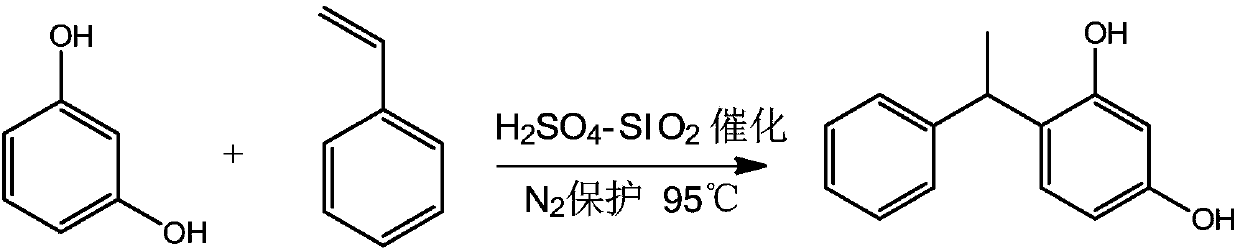
[0025] Example 1 is based on H 2 SO 4 -SiO 2 The method suitable for industrial preparation of phenylethyl resorcinol of solid acid catalyst
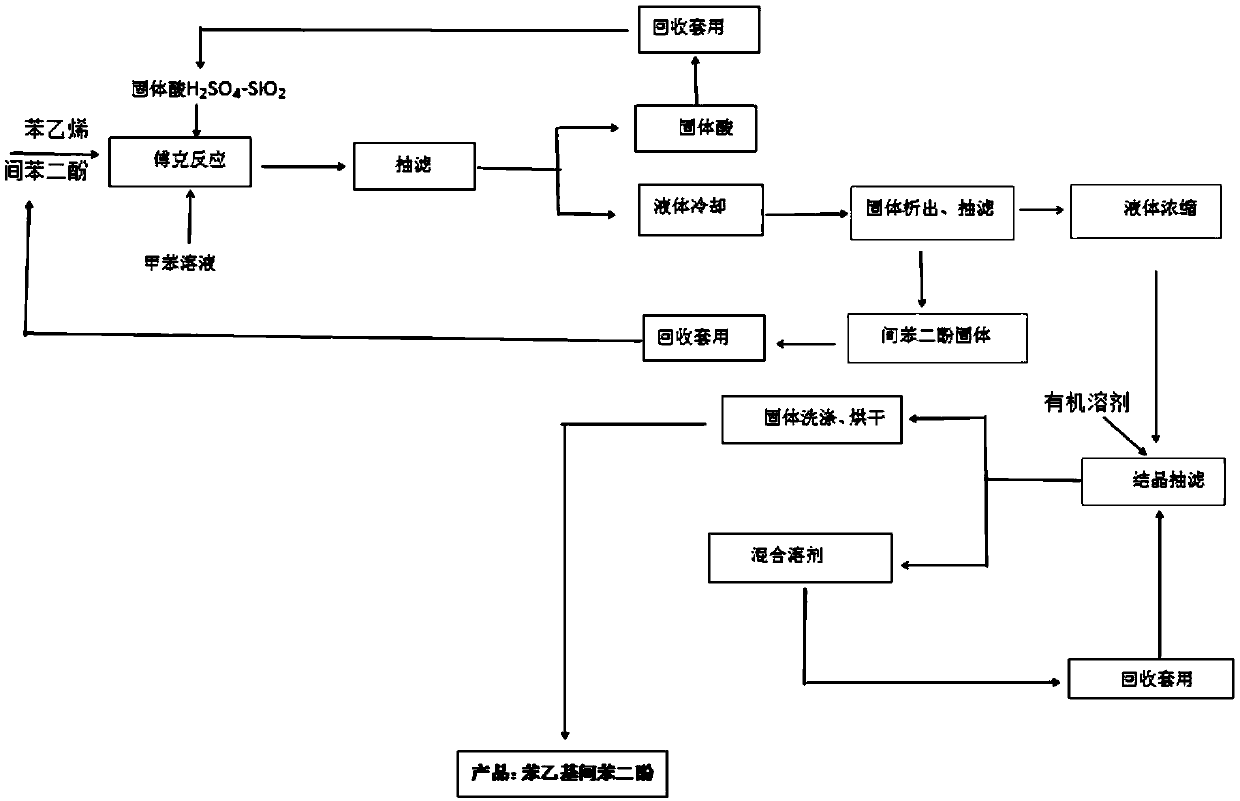
[0026] process such as figure 1 As shown, the method is as follows:
[0027] (1) Add 50L of toluene to the reactor, start stirring, add 31.7kg (288mol) of resorcinol (mw: 110.11), 0.15kg of catalyst solid acid H 2 SO 4 -SiO 2 (by mass ratio H 2 SO 4 :SiO2 2 =1:1), after airtight, vacuumize, nitrogen replacement 3 times, under the protection of nitrogen, raise the temperature of the reactor to 95°C, after the temperature is stable, slowly drop 15kg (144mol) of styrene, control the feeding time 1.5 -2 hours, heat preservation reaction for 3 hours, the liquid phase detection reaction is complete, the reaction is stopped, and the yield is 84%.
[0028] (2) Add 10 liters of toluene to the product obtained in step (1), and reclaim the solid acid H by hot filtration 2 SO 4 -SiO 2 Catalyst, set aside, the filtrate was slowly cooled...
Embodiment 2
[0033] (1) The parameters of the design "three factors and two levels" reaction conditions are shown in Table 1, the method is the same as in Example 1, and the results are shown in Table 2.
[0034] Table 1
[0035] level factor
Resorcinol:styrene molar ratio
solid acid H 2 SO 4 -SiO 2 Addition mass%
temperature reflex
1
1:1
0.5
65
2
2.5:1
5
110
[0036] Table 2
[0037]
[0038] As can be seen from Table 1 and Table 2, through the "three factors and two levels" orthogonal experimental design, the priority order of key reaction conditions is: resorcinol: styrene molar ratio > solid acid H 2 SO 4 -SiO 2 Addition amount>reaction temperature.
Embodiment 3
[0040] (1) Add 50L of toluene to the reactor, start stirring, add 39.6kg (360mol) of resorcinol (mw: 110.11), 0.45kg of catalyst solid acid H 2 SO 4 -SiO 2 (by mass ratio H 2 SO 4 :SiO2 2 =1:1), after airtight, vacuumize, nitrogen replacement 3 times, under the protection of nitrogen, the reaction kettle is heated up to 95 ℃, after the temperature is stable, slowly drop 15kg (144mol) of styrene (mw: 104.15) , control the feeding time for 1.5-2 hours, and keep the temperature for 3 hours to react. After the liquid phase detection, the reaction is complete, the yield is 89%, and the reaction is stopped.
[0041] (2) Add 10 liters of toluene to the reaction solution, reclaim the solid acid H by hot filtration 2 SO 4 -SiO 2 Catalyst, the filtrate was slowly cooled to 0°C under stirring conditions, solid resorcinol was precipitated, filtered, and the solid resorcinol was washed and recovered with 12 liters (6 liters×2 times) of toluene for subsequent use. Concentrate the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com