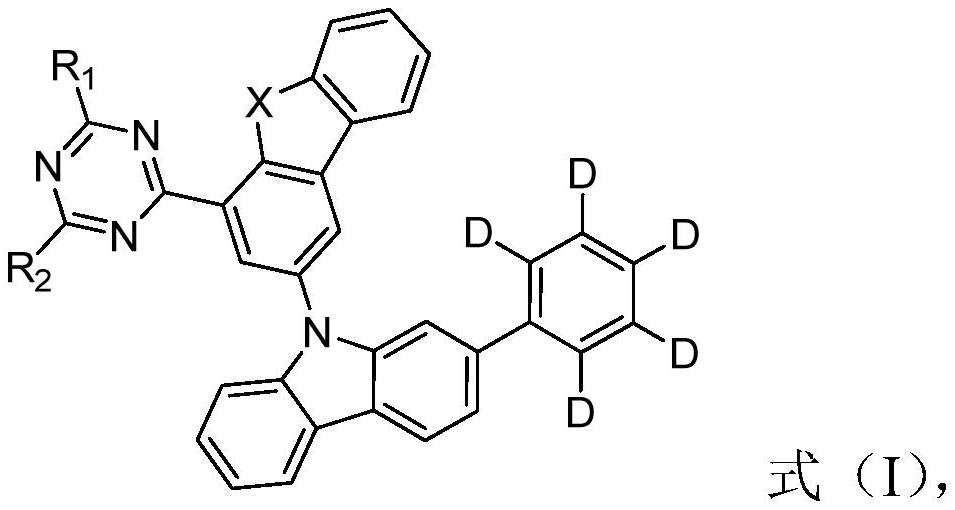
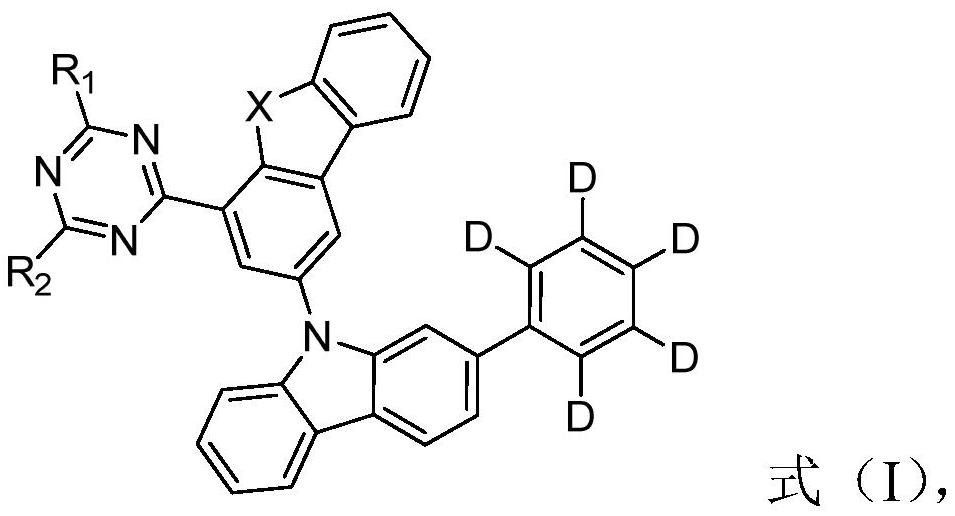
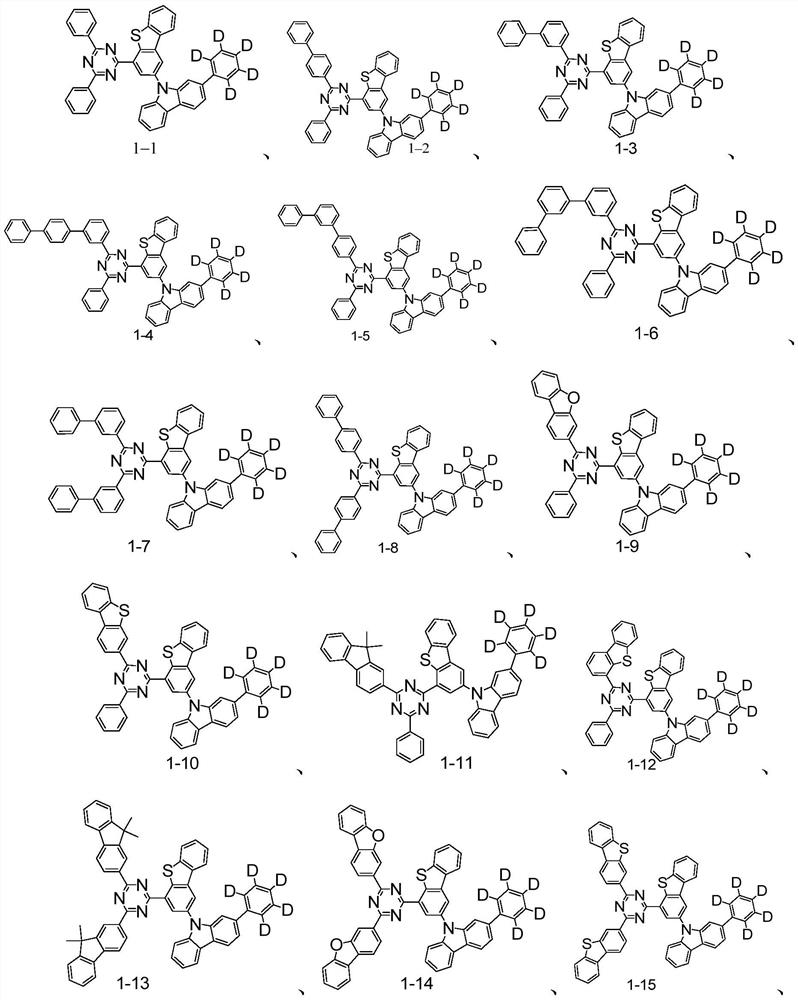
Triazine compounds containing deuterated phenyl groups and their applications and organic electroluminescent devices
A technology of triazine compound and deuterated benzene, applied in the field of organic electroluminescence devices, can solve the problems of voltage, efficiency and service life not meeting the requirements, device performance indicators not meeting the use requirements, etc., so as to improve the utilization rate and reduce the Effect of energy loss and improvement of current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080]The preferred solvent for the preparation of organic electroluminescent devices of the present invention is selected from toluene, anisole, o-xylene, m-xylene, p-xylene, methyl benzoate, mesitylene, tetralin, ortho-dimethoxy Benzene, THF, methyl-THF, THP, chlorobenzene, phenoxytoluene, especially 3-phenoxytoluene, 1,2,3,5-tetramethylbenzene, 1,2,4,5-tetramethylbenzene Methylbenzene, 1-methylnaphthalene, 2-methylbenzothiazole, 2-phenoxyethanol, 2-pyrrolidone, 3-methylanisole, 4-methylanisole, 3,4-di Methyl Anisole, 3,5-Dimethyl Anisole, Acetophenone, Benzothiazole, Butyl Benzoate, Isopropanol, Cumene, Cyclohexanol, Cyclohexanone, Cyclohexylbenzene, Decalin, dodecylbenzene, methyl benzoate, NMP, p-methylisocyanobenzene, phenetole, 1,4-diisopropylbenzene, dibenzyl ether, diethylene glycol butyl methyl ether, Triethylene glycol butyl methyl ether, diethylene glycol dibutyl ether, triethylene glycol dibutyl ether, diethylene glycol monobutyl ether, tripropylene glycol dimeth...
preparation example 1
[0093] Preparation example 1: the compound shown in preparation formula (1-1)
[0094]
[0095]
[0096] Synthesis of intermediate 1-1-1: Dissolve 0.1mol of 1-aminodibenzothiophene in 200ml of acetic acid, heat up to 50°C, add 0.06mol of potassium iodide, then add 0.03mol of potassium iodate in three batches, add the second After one batch, the reaction solution turns red, and then the next batch of potassium iodate is added when it changes from red to white, and the degree of conversion of the reaction is monitored. After the reaction is completed, the intermediate 1-1-1 is obtained by suction filtration (yield 45%) ).
[0097] Calculated value C12H8INS: 325.17±1.1H-NMR (400MHz, CDCl 3 )(ppm) δ=6.27~6.28(2H, s), 6.65~6.66(1H, d), 7.35~7.36(1H, d), 7.50~7.52(2H, m), 7.98~7.99(1H, m) , 8.45-8.46 (1H, m).
[0098] Synthesis of Intermediate 1-1-2: Dissolve 0.045mol of Intermediate 1-1-1 in 150ml of N,N-dimethylformamide, raise the temperature to 50°C, add dropwise 50ml o...
preparation example 2
[0108] Preparation example 2: the compound shown in preparation formula (1-2)
[0109]
[0110] Synthesis of Intermediate 1-2-1: The synthesis method was the same as that of Intermediate 1-1-4 to obtain Intermediate 1-2-1 (53% yield).
[0111] Calculated value C33H20BrN3S: 570.50±1.1H-NMR (400MHz, CDCl 3 )(ppm) δ=7.25~7.26(2H, m), 7.41~7.52(10H, m), 7.67~7.67(1H, s), 7.85~7.88(3H, m), 7.98~7.99(1H, m) , 8.28~8.29 (2H, m), 8.45~8.46 (1H, m).
[0112] Synthesis of compound 1-2: the synthesis method was the same as that of compound 1-1 to obtain compound 1-2 (yield 60%).
[0113] Calculated value C33H20BrN3S: 570.50±1.1H-NMR (400MHz, CDCl3 )(ppm)δ=7.25~7.52(14H, m), 7.62~7.62(1H, s), 7.69~7.69(1H, s), 7.79~7.98(6H, m), 8.18~8.19(1H, m) , 8.28~8.29(2H, m), 8.45~8.46(1H, m), 8.55~8.56(1H, m)
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com