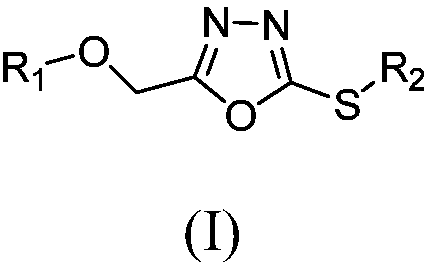
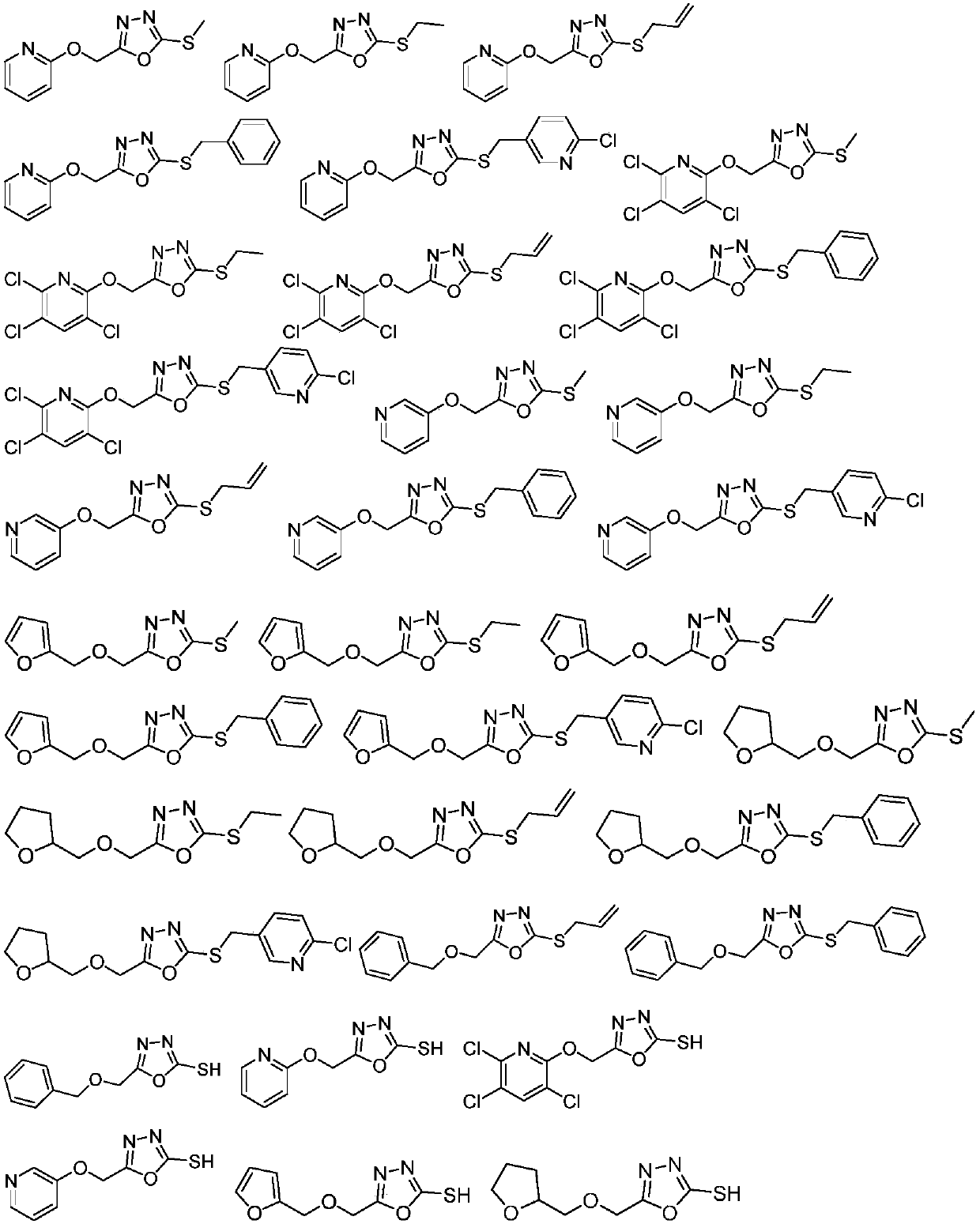
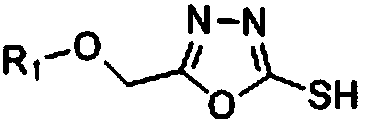
1,3,4-oxadiazole thioether compound as well as preparation method and application thereof
A technology for oxadiazole sulfide and compound, which is applied in the field of 1,3,4-oxadiazole sulfide compound and its preparation, can solve the problems of citrus rot and influence on citrus yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of 2-(pyridine-2-hydroxyl) ethyl acetate
[0057] Dissolve 8.0g (84.1mmol) of 2-hydroxypyridine in 100mL of acetone, then add potassium carbonate (14.0g, 100.9mmol) and ethyl bromoacetate (9.4mL, 84.2mmol), react at 56°C for 12h to stop the reaction, add 100mL Ethyl acetate, washed with water 30 mL x 3 times, dried over anhydrous sodium sulfate, and precipitated to obtain 11.7 g of a colorless oil with a yield of 76.7%.
[0058] For other ester intermediate compounds, corresponding raw materials or substituents are used, and are synthesized with reference to the steps of Example 1.
Embodiment 2
[0059] Embodiment 2: the preparation of 2-(2-pyridyl-oxygen) acetylhydrazide
[0060] Dissolve 11.5g (106.7mmol) of ethyl 2-(pyridine-2-hydroxy)acetate in 100mL of absolute ethanol, then add hydrazine hydrate (8.5mL, 138.6mmol), react at 85°C for 6h to stop the reaction, and desolvate column chromatography (Eluant dichloromethane:methanol=5:1) 10.2 g of yellow oil, yield 57.2%.
[0061] Other hydrazide intermediate compounds are synthesized by referring to the steps of Example 2 using corresponding raw materials or substituents.
Embodiment 3
[0062] Example 3: Preparation of 2-thiol-(5-(2-pyridyl-oxygen)-methyl)-1,3,4-oxadiazole
[0063] Dissolve 9.9g (59.4mmol) of 2-(2-pyridyl-oxy)acetylhydrazide in 100mL of absolute ethanol, then add potassium hydroxide (7.8g, 118.9mmol) and carbon disulfide (5.4mL, 89.2mmol), and react at room temperature 12h, react at 85°C for 12h to stop the reaction, add 100mL of water after precipitation, adjust Ph=3-4 with 5% hydrochloric acid aqueous solution, filter with suction to obtain 4.1g of light yellow solid, yield 33.3%, melting point: 184-185°C.
[0064] Other thiol intermediate compounds are synthesized with reference to the steps of Example 3 using corresponding raw materials or substituents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com