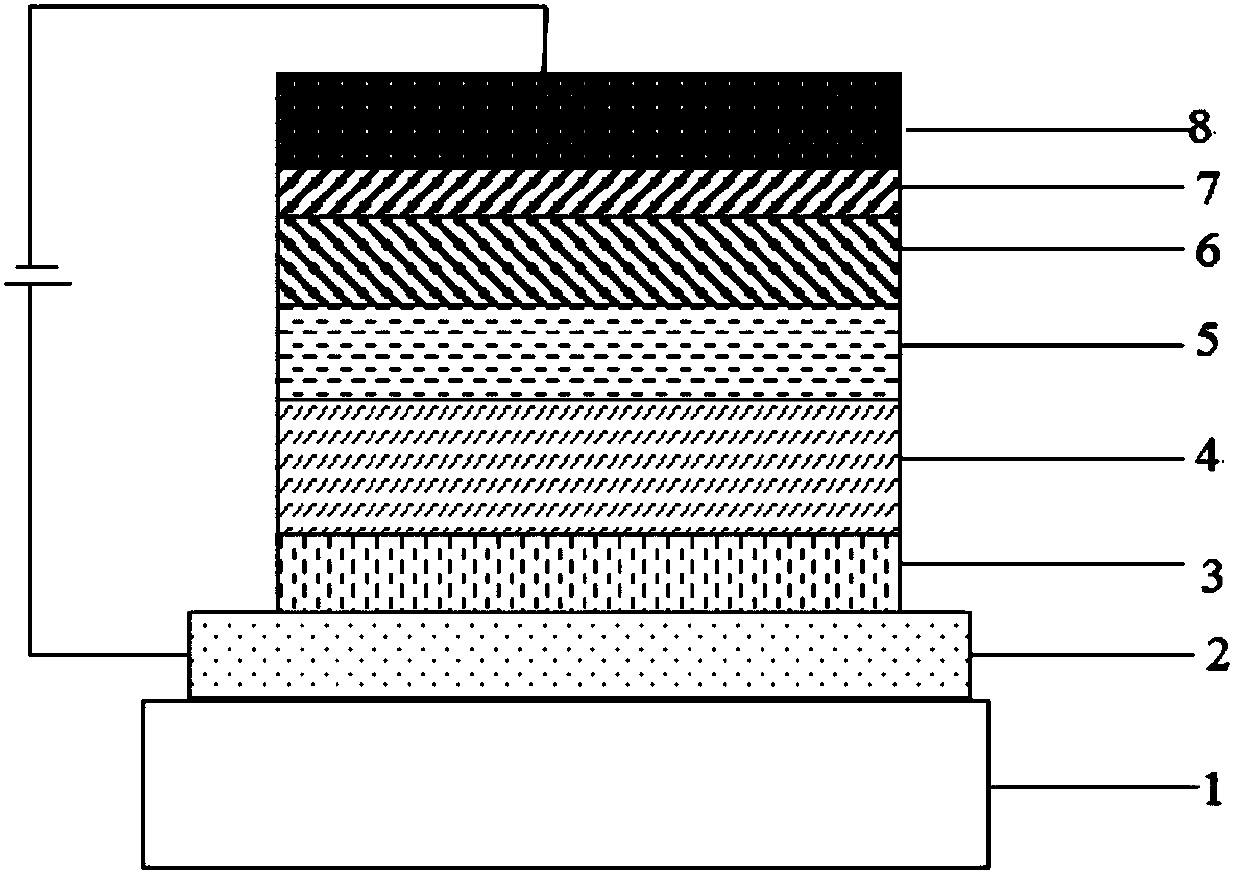
Boron-containing organic compound and application thereof in organic electroluminescent device
An organic compound and luminescent technology, applied in the field of semiconductors, can solve the problems of efficiency roll-off, high exciton utilization rate, high fluorescence radiation efficiency, low S1 state radiation transition rate, etc., and achieve good photoelectric performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The synthesis of embodiment 1 compound 5
[0057] The synthetic steps of compound are as follows:
[0058]
[0059] In a 150ml four-neck flask, add 0.01mol of A1 and 50ml of tetrahydrofuran, protect it with nitrogen, cool to -78 degrees Celsius, and then slowly drop in 0.02mol of n-butyl lithium cyclohexane solution. After the drop is completed, stir for 3 hours. Raise the temperature to -10 degrees Celsius, drop in 0.01mol dimethyl tin dichloride tetrahydrofuran solution, after the dropwise addition, keep the temperature for reaction for 1 hour, then warm up to room temperature, remove the tetrahydrofuran by rotary evaporation, and then rinse twice with ethanol to obtain Pale yellow solid B1, purity 98.2%, yield 90%. HRMS(m / z): [M+H] + , the theoretical value is 417.14, and the measured value is 417.11.
[0060] Add 50ml of toluene and 0.01mol B1 to a 150ml three-necked flask, protect it with nitrogen, cool down to -78 degrees Celsius, and then slowly drop in a t...
Embodiment 2
[0063] The synthesis of embodiment 2 compound 10
[0064] The synthetic steps of compound are as follows:
[0065]
[0066] 150ml four-neck flask, add 0.01mol of A2 and 50ml of tetrahydrofuran, nitrogen protection, cool to -78 degrees Celsius, then slowly drop into 0.02mol of n-butyllithium cyclohexane solution, after the completion of the drop, stir the reaction for 3 hours, Raise the temperature to -10 degrees Celsius, drop in 0.01mol dimethyl tin dichloride tetrahydrofuran solution, after the dropwise addition, keep the temperature for reaction for 1 hour, then warm up to room temperature, remove the tetrahydrofuran by rotary evaporation, and then rinse twice with ethanol to obtain Pale yellow solid B2 with a purity of 98.4% and a yield of 89.5%. HRMS(m / z): [M+H] + , the theoretical value is 467.20, and the measured value is 467.15.
[0067] Add 50ml of toluene and 0.01mol B2 to a 150ml three-necked flask, protect it with nitrogen, cool down to -78 degrees Celsius, th...
Embodiment 3
[0070] The synthesis of embodiment 3 compound 20
[0071] The synthetic steps of compound are as follows:
[0072]
[0073] Add 0.01mol of A3 and 50ml of tetrahydrofuran into a 150ml four-necked flask, protect it with nitrogen, cool to -78 degrees Celsius, then slowly drop in 0.02mol of n-butyl lithium cyclohexane solution, and stir for 3 hours to react. Raise the temperature to -10 degrees Celsius, drop in 0.01mol dimethyl tin dichloride tetrahydrofuran solution, after the dropwise addition, keep the temperature for reaction for 1 hour, then warm up to room temperature, remove the tetrahydrofuran by rotary evaporation, and then rinse twice with ethanol to obtain Pale yellow solid B3 with a purity of 97.6% and a yield of 87.5%. HRMS(m / z): [M+H] + , the theoretical value is 391.06, and the measured value is 391.12.
[0074] Add 50ml of toluene and 0.01mol B3 into a 150ml three-necked flask, protect it with nitrogen, cool down to -78 degrees Celsius, and then slowly drop i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com