Shell nacre matrix protein, preparation method and use thereof
A technology of matrix protein and nacre, which is applied in the field of medicine, can solve the problems of easy loss of protein activity, severe extraction conditions, and loss of active ingredients, and achieve the effect of promoting mineralization activity, high matrix protein concentration, and short duration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 EDTA dissolves nacre powder experiment
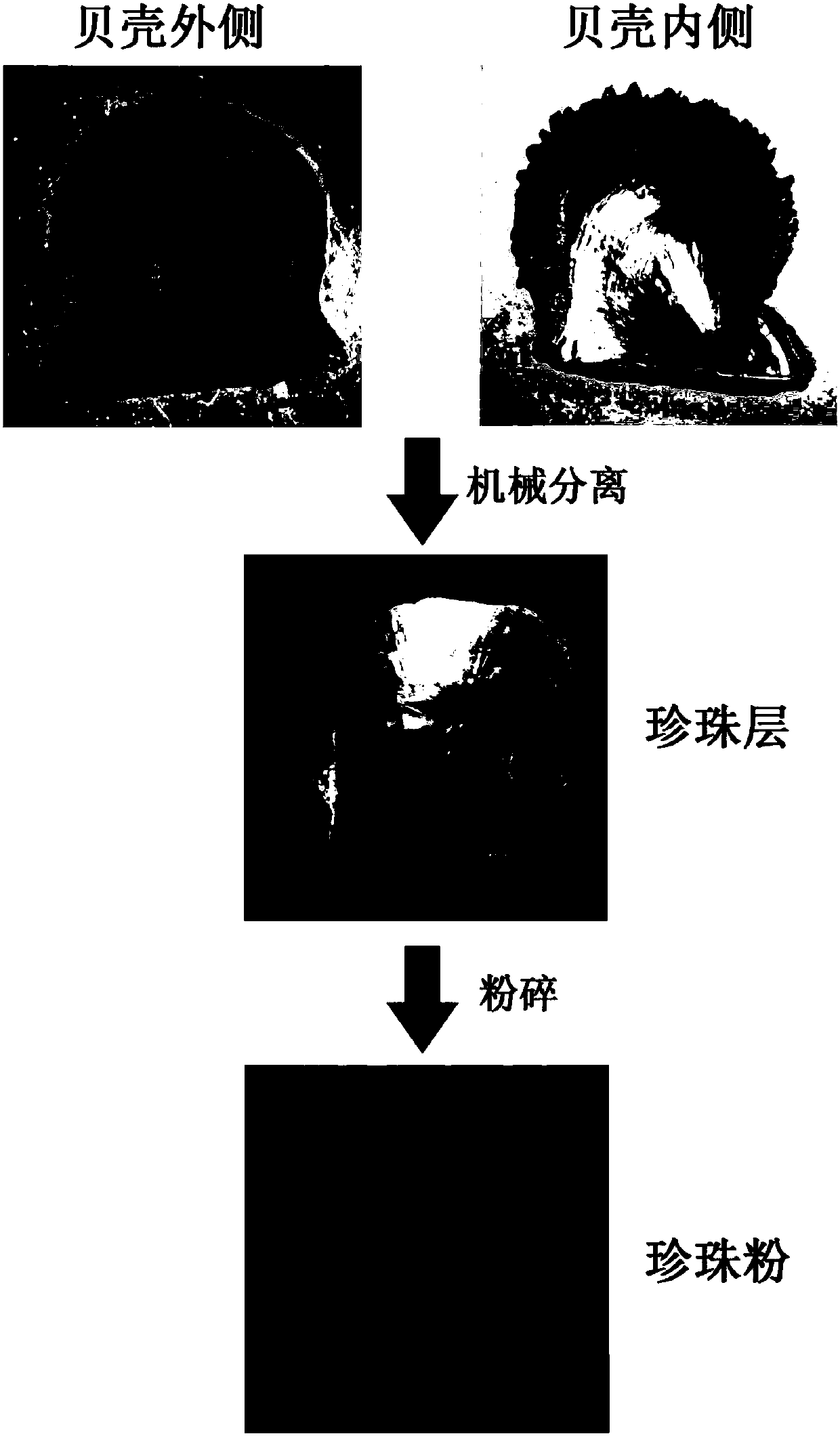
[0048] The Hepu pinnacle was sacrificed after one week of acclimatization in the laboratory to obtain its shells. Then use scissors to cut off the edge of the shell, and use a file to mechanically scrape the remaining shell to remove the outer prismatic layer and cuticle to obtain the shell nacre sample. The specific steps of EDTA dissolving nacre powder are as follows:
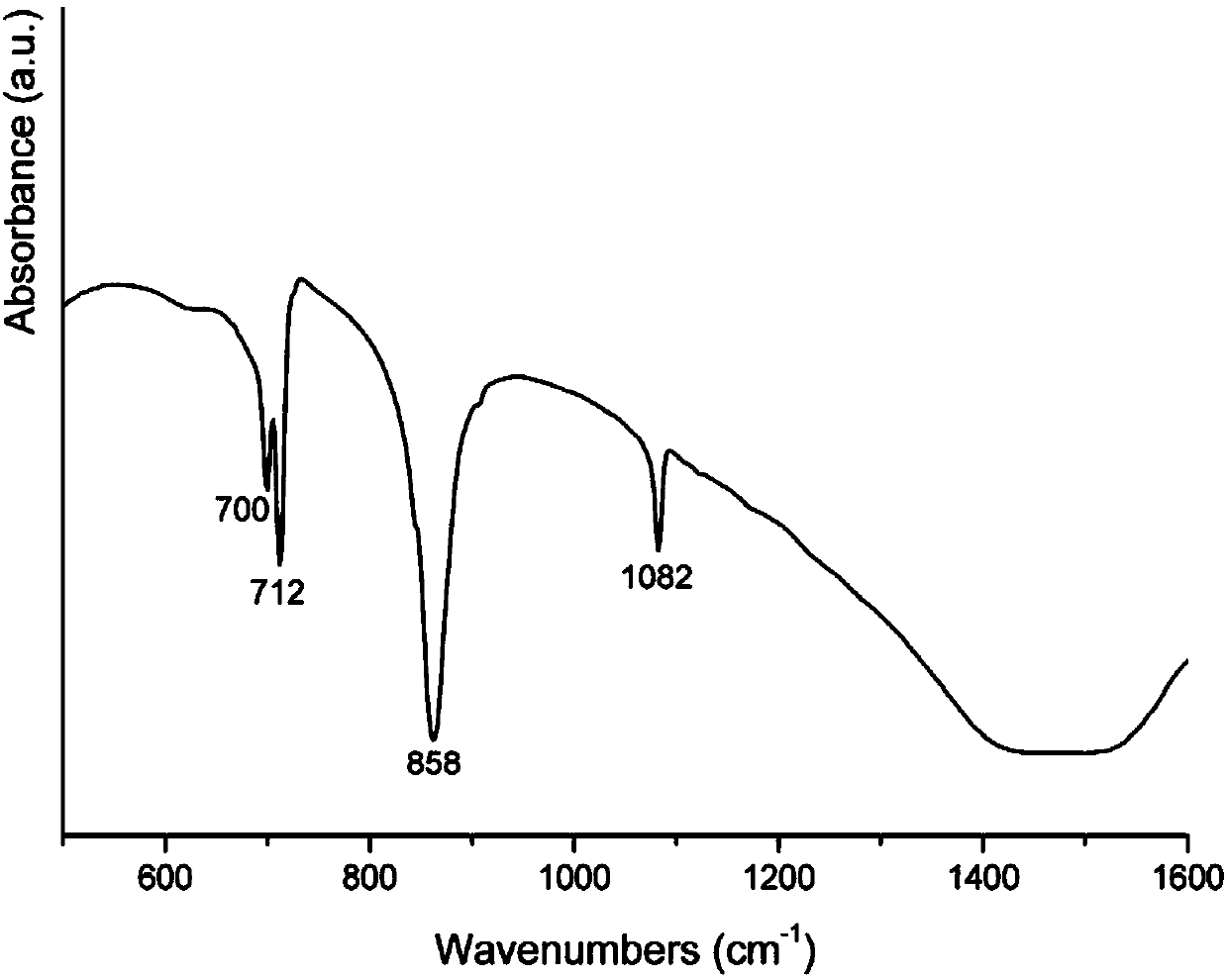
[0049] ① Pulverize the prepared nacre sample with a high-speed pulverizer, and screen powders with different particle sizes (60 mesh = 250 μm, 180 mesh = 80 μm) with different screens to obtain particle sizes ≤ 80 μm, 80 μm < particle size < 250 μm, and For powders with a particle size ≥ 250 μm, nacre powders are identified by FTIR spectroscopy;
[0050] ②Put the nacre powder into 0.5M EDTA (dissolve every 10g of powder in 100ml of 0.5M EDTA, pH8.0) and stir to dissolve, observe the dissolution time.
Embodiment 2
[0051] The extraction of embodiment 2 conch nacre matrix protein
[0052] The shells came from Hepu pearl oysters in the sea area of Daya Bay, Shenzhen, Guangdong. They were sacrificed after one week of acclimatization in the laboratory to obtain their shells. Then use scissors to cut off the edge of the shell, and use a file to mechanically scrape the remaining shell to remove the outer prismatic layer and cuticle to obtain the shell nacre sample. The specific steps of extraction of EDTA soluble shell matrix protein are as follows:
[0053] ① The prepared nacre sample is crushed into a powder with a diameter of less than 80 μm by a high-speed pulverizer, and the nacre powder is identified by FTIR spectroscopy;
[0054] ②Put the nacre powder into 0.5M EDTA (10g / 100ml) and stir to dissolve for 36 hours;
[0055] ③ Centrifuge at 13,000 rpm for 30 minutes at 4°C. Repeat this step twice to fully remove undissolved impurities in EDTA;
[0056] ④Concentrate the obtained superna...
Embodiment 3
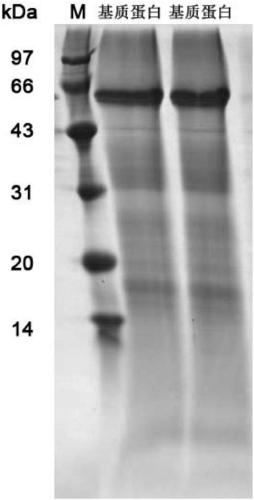
[0058] Example 3 SDS-PAGE protein size distribution identification
[0059] ① Prepare the protein powder with ultrapure water to make a 3mg / ml protein solution, centrifuge at 25000rpm for 15min at 4°C, take the supernatant, and drain the supernatant;
[0060] ②Merge the dried supernatant and precipitate, add 200 μl of PMSF with SDS L3, final concentration of 1mM, and 2mM EDTA, mix well, place on ice for 5min, add final concentration of 10mM DTT (PMSF, EDTA, DTT) The added volume is 1% of the protein solution volume);
[0061] ③ Sonicate on ice for 5 minutes (work 5s, interval 5s, power 100W), 4°C, 25000rpm centrifuge 20min;
[0062] Transfer the supernatant to a new 1.5ml centrifuge tube, add a final concentration of 10mM DTT (1% of the volume of the protein solution), and bathe in water at 56°C for 1h;
[0063] ④ Cool to room temperature, add a final concentration of 55mM IAM (1% of the volume of the protein solution), and place in a dark room for 45min;
[0064] ⑤ Centrif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com