a cofe 2 o 4 Preparation method and application of nanosheet oxygen evolution catalyst
A nanosheet and catalyst technology, applied in the field of energy material electrocatalysis, achieves the effects of simple equipment, low source cost and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Add potassium cobalt cyanide, ferric nitrate and cobalt oxalate into water at a molar ratio of 1:2:1 to form an aqueous solution. The amount of water added is just enough to dissolve the three raw materials. Stir at room temperature for 4.5 hours, then statically Place the precipitate for 4 hours, and wash the obtained complex precipitate with water and ethanol three times in sequence to obtain an iron-cobalt-based precursor.
[0038] Add 6M NaBH dropwise to the above iron-cobalt-based precursor 4 Aqueous solution until bubbles are no longer generated, the dropping rate is 20 drops / min, the resulting black liquid is left to stand, centrifuged to obtain a black solid, and the black solid is washed three times with water and ethanol respectively to obtain CoFe 2 o 4 Nanosheet Oxygen Evolution Catalyst.
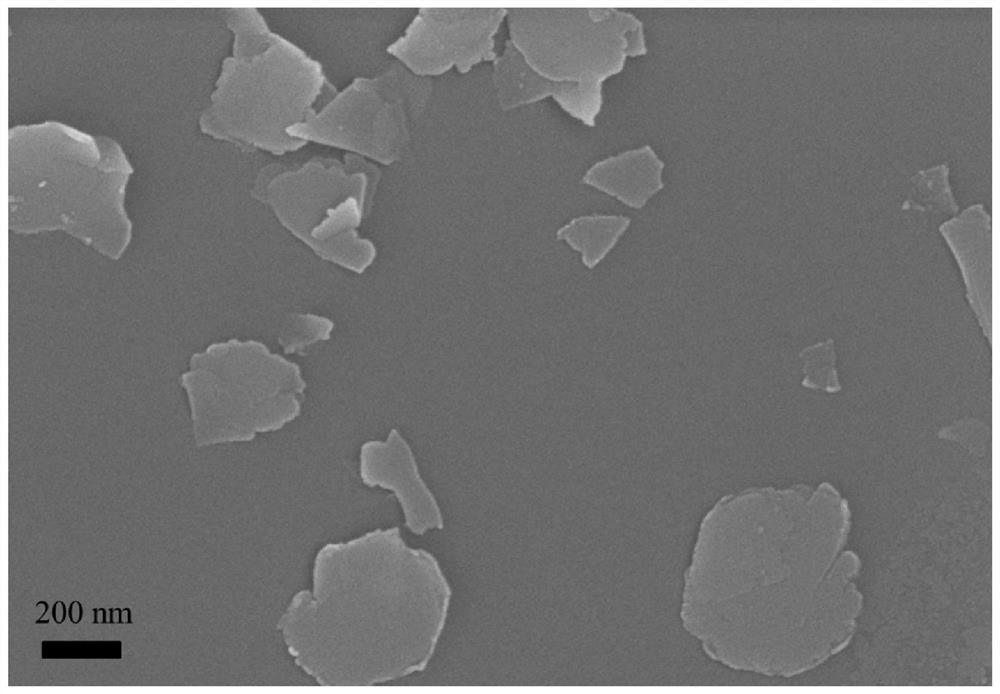
[0039] figure 1 for CoFe 2 o 4 The SEM photo of the nanosheet oxygen evolution catalyst shows that the catalyst is a sheet structure with an average particle size of ...
Embodiment 2
[0046] Add potassium ferricyanide, ferric oxalate and cobalt nitrate into water according to the molar ratio of 1:3:1 to form an aqueous solution. The amount of water added is just enough to dissolve the three raw materials. Stir at room temperature for 4.5 hours, then statically Place the precipitate for 4 hours, and wash the obtained complex precipitate with water and ethanol three times in sequence to obtain an iron-cobalt-based precursor.
[0047] Add 6M NaBH dropwise to the above iron-cobalt-based precursor 4 Aqueous solution until bubbles are no longer generated, the dropping rate is 21 drops / min, the obtained black liquid is left standing, centrifuged to obtain a black solid, and the black solid is washed three times with water and ethanol respectively to obtain CoFe 2 o 4 Nanosheet Oxygen Evolution Catalyst.
Embodiment 3
[0049] Add potassium ferrocyanide, cobalt sulfate and ferric chloride into water according to the molar ratio of 2:2:1 to form an aqueous solution. The amount of water added is just enough to dissolve the three raw materials. Stir at room temperature for 4.5 hours. After standing for precipitation for 4 hours, the obtained complex precipitate was washed with water and ethanol three times in sequence to obtain an iron-cobalt-based precursor.
[0050] Add 6M NaBH dropwise to the above iron-cobalt-based precursor 4 Aqueous solution until bubbles are no longer generated, the dropping rate is 20 drops / min, the resulting black liquid is left to stand, centrifuged to obtain a black solid, and the black solid is washed three times with water and ethanol respectively to obtain CoFe 2 o 4 Nanosheet Oxygen Evolution Catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com