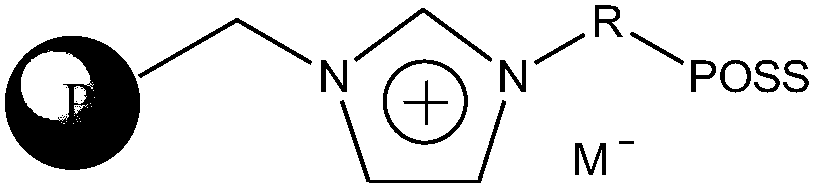
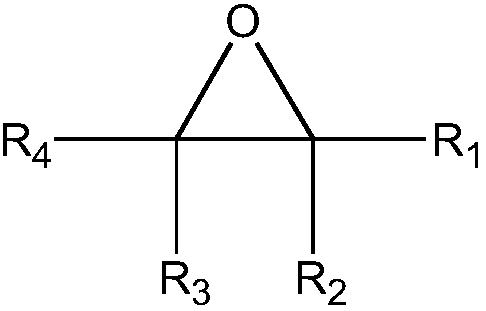
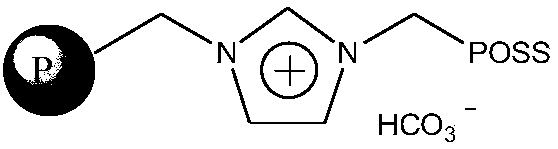
Method of catalytically hydrating alkylene oxide to produce ethylene glycol
A technology for the catalytic hydration of alkylene oxide and alkylene oxide, which is applied in the production of bulk chemicals, chemical instruments and methods, preparation of organic compounds, etc. Harsh and other problems, to achieve the effect of increasing the glass transition temperature, good heat resistance, and high swelling resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] [Example 1] Preparation of Ion Exchange Resin Catalyst
[0036] Add 47.0 grams of styrene, 2.3 grams of divinylbenzene and 1.6 grams of benzoyl peroxide initiator in a 500 milliliter three-necked flask, stir and react at 60°C for 2.0 hours; then add 0.6 grams of multi-walled carbon nanotubes and continue stirring Prepolymerization was carried out for 1 hour. 260 mL of deionized water in which 2.0 g of gelatin had been dissolved was added. Adjust the stirring speed, and at the same time gradually raise the temperature to 80°C, and react for 5 hours; then raise the temperature to 90°C, react for 5 hours, and finally raise the temperature to 98°C, and react for 6 hours. After the reaction, pour out the upper liquid, wash with 85°C hot water, then wash with cold water, then filter, put it in an oven to dry at 80°C, sieve, and collect the composite gel with a particle size within the range of 0.35-0.60mm Microsphere A1.
[0037] Chloromethylation of composite gel microsph...
Embodiment 2
[0042] [Example 2] Preparation of Ion Exchange Resin Catalyst
[0043] Add the monomer mixture solution (60.0 gram styrene, 1.0 gram divinylbenzene, 1.6 gram multi-walled carbon nanotubes and 1.0 gram benzoyl peroxide that contains initiator in 500 milliliters three-necked flask, this solution is prior to 70 ℃ Stir the reaction for 0.5 hours), start the stirrer, add a mixed solution of 200 ml deionized water and 4 grams of polyvinyl alcohol, heat up to 85°C, react for 3 hours, then heat up to 90°C, react for 9 hours, and finally heat up to 100°C , reacted for 10 hours. After the reaction, pour out the upper liquid, wash with 85°C hot water, then wash with cold water, then filter, put it in an oven to dry at 80°C, sieve, and collect the composite gel with a particle size within the range of 0.35-0.60 mm Microsphere B1.
[0044] Chloromethylation of composite microspheres: in a 500-ml three-necked flask, add 50 grams of composite microspheres B1 and 200 milliliters of chloroet...
Embodiment 3
[0049] [Example 3] Preparation of Ion Exchange Resin Catalyst
[0050] Add the monomer mixture solution (42.5 grams of styrene, 2.5 grams of divinylbenzene, 0.1 gram of multi-walled carbon nanotubes and 2.0 grams of benzoyl peroxide containing the initiator in a 500 milliliter three-necked flask, the solution was heated at 70° C. Stirring reaction for 1.5 hours), add a mixed solution of 200 ml deionized water and 4 grams of polyvinyl alcohol, heat up to 85°C, react for 3 hours, then heat up to 90°C, react for 9 hours, finally heat up to 100°C, react for 10 hours . After the reaction, pour out the upper liquid, wash with 85°C hot water, then wash with cold water, then filter, put it in an oven to dry at 80°C, sieve, and collect the composite gel with a particle size within the range of 0.35-0.60 mm Microsphere C1.
[0051] Chloromethylation of composite microspheres: In a 250 ml three-necked flask, add 20 g of composite microspheres C1 and 100 ml of 1,4-dichloromethoxybutane,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com