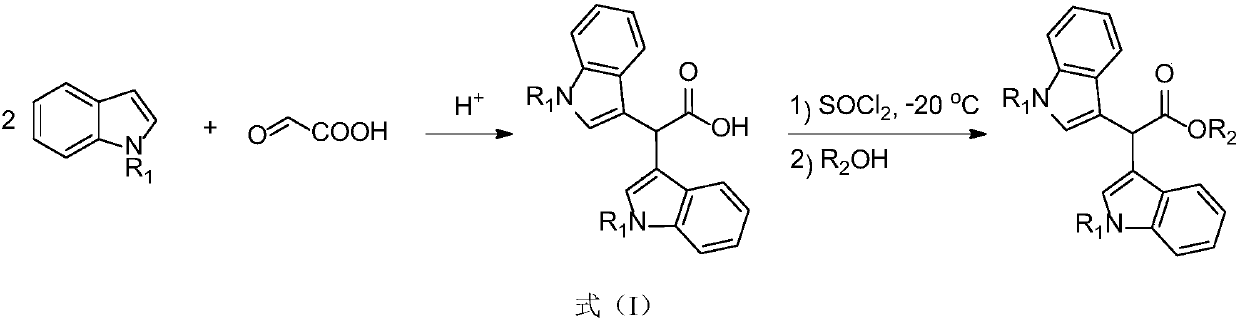
Preparation method of 3,3'-diindolyl acetate
A technology of indole acetate and indole derivatives, which is applied in the field of preparation of 3,3'-diindole acetate compounds, can solve the problems of harsh reaction conditions, high toxicity in use, and cumbersome steps, etc. Achieve the effects of less reaction steps, easy operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of 3,3'-diindole ethyl acetate
[0037]
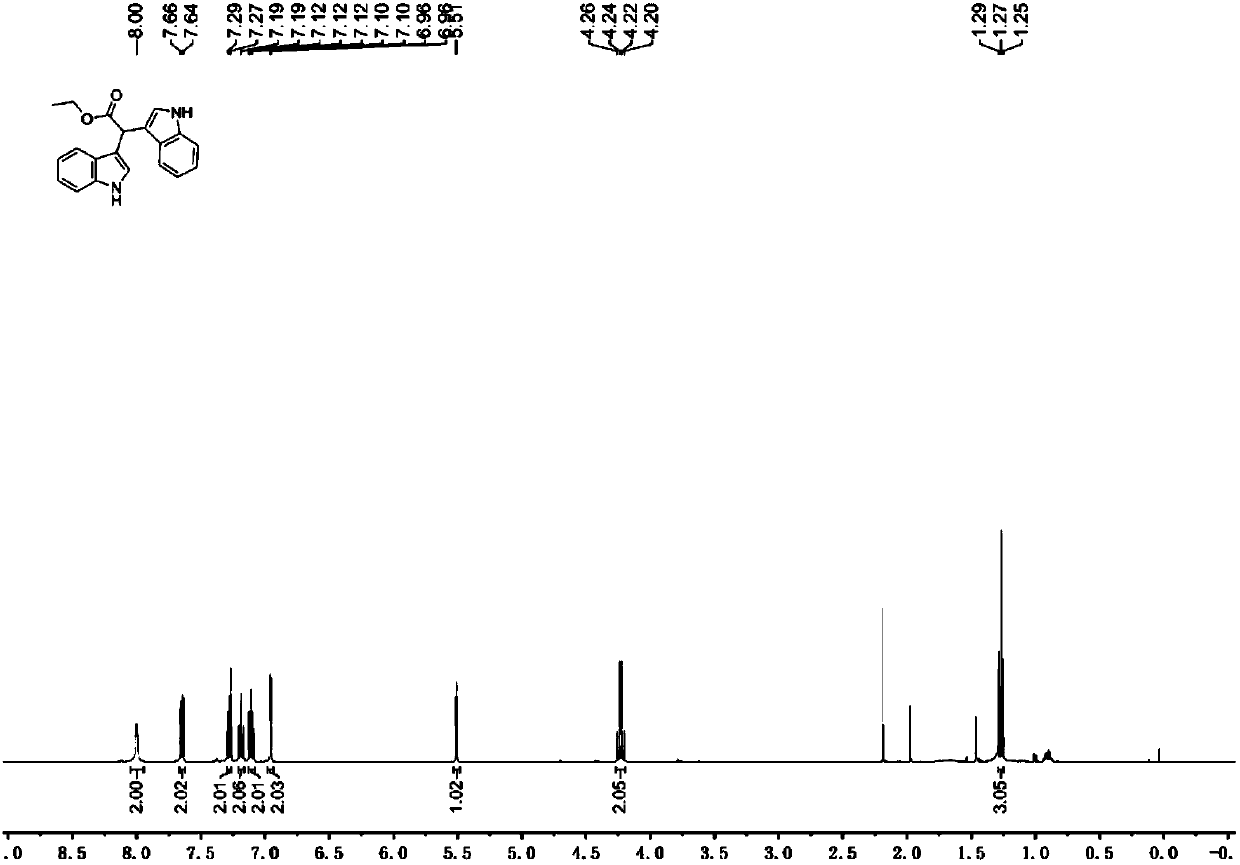
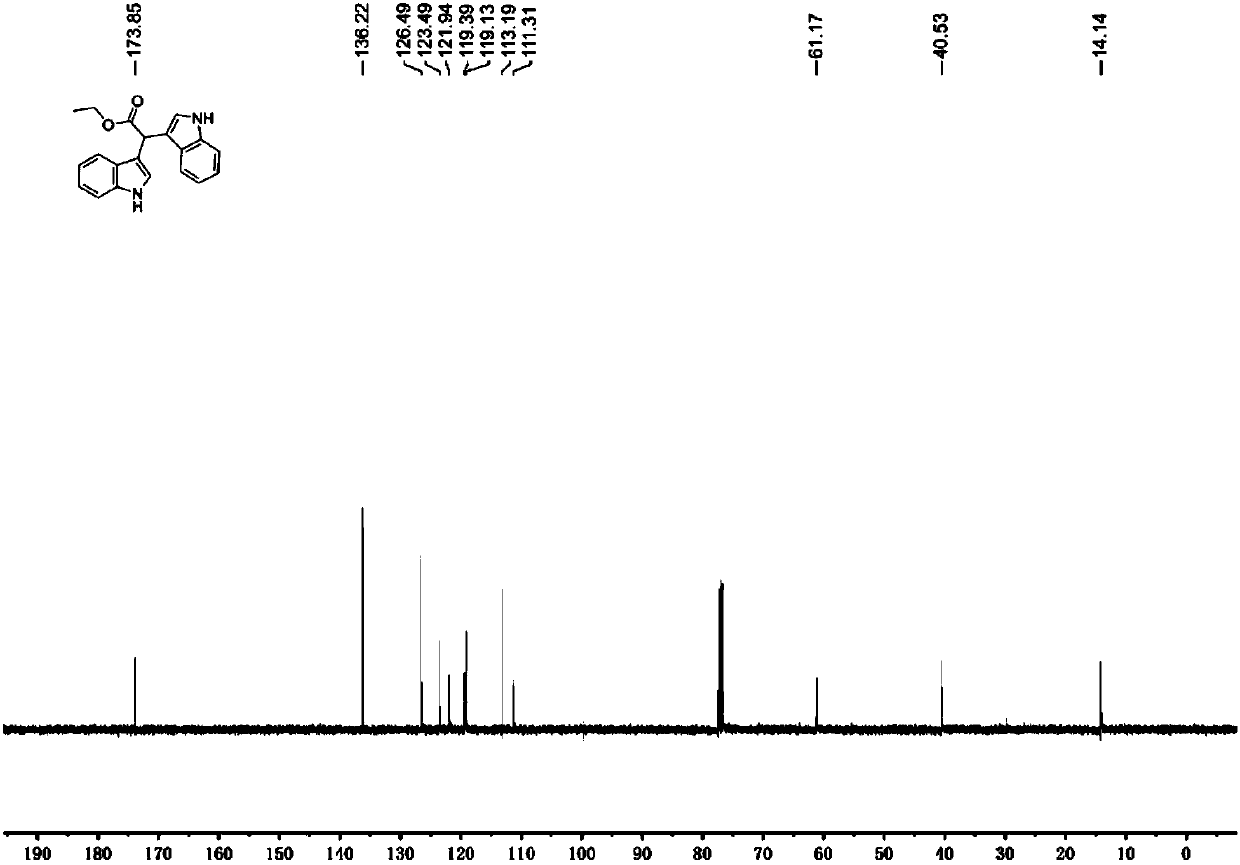
[0038] In a 25 mL flask, dissolve 193 mg (1 mmol) (4-methylphenyl) ethyl aminoacetate in 5.0 mL ethanol, then add 246 mg (2.1 mmol) indole, 6.9 mg (1 mol%) eosin Y, 0.11mL (1.5eq) of trifluoroacetic acid was stirred at room temperature for 12 hours under the irradiation of a 26W energy-saving lamp. Then add saturated sodium bicarbonate solution to quench the reaction, extract with ethyl acetate, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, column chromatography (petroleum ether / ethyl acetate=4:1) to obtain 312mg of product, yield 98 %. Pale red solid. 1 H NMR (400MHz, CDCl 3 ): δ8.00(s,2H),7.65(d,J=8.0Hz,2H),7.28(d,J=8.2Hz,2H),7.19(m,2H),7.10(m,2H),6.96 (d, J=2.4Hz, 2H), 5.51(s, 1H), 4.23(q, J=7.1Hz, 2H), 1.27(t, J=7.1Hz, 3H). 13 C NMR (100MHz, CDCl 3 ): δ173.9, 136.2, 126.5, 123.5, 121.9, 119.4, 119.1, 113.2, 111.3, 61.2, 40.5, 14.1.
Embodiment 2
[0039] Embodiment 2: the preparation of 3,3'-diindole acetic acid benzyl ester
[0040]
[0041] In a 25mL flask, dissolve 271mg (1mmol) benzyl (4-methoxyphenyl)aminoacetate in 5.0mL 1,2-dichloroethane, then add 246mg (2.1mmol) indole, 10.2mg ( 1mol%) rose bengal, 0.087mL (1.5eq) phosphoric acid, and stirred at room temperature for 10 hours under the irradiation of a 5W blue LED light. Then add saturated sodium bicarbonate solution to quench the reaction, extract with ethyl acetate, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, column chromatography (petroleum ether / ethyl acetate=4:1) to obtain 346mg product, yield 91 %. Pale yellow solid. 1 H NMR (400 MHz, acetone-d 6):δ7.95(s,2H),7.50(d,J=7.6Hz,2H),7.29-6.97(m,13H),5.51(s,1H),5.11(s,2H). 13 C NMR (100MHz, acetone-d 6 ): δ172.7, 136.9, 136.5, 128.2, 128.0, 127.8, 126.9, 123.6, 121.3, 119.1, 118.7, 113.1, 111.3, 66.0, 40.7.
Embodiment 3
[0042] Embodiment 3: Preparation of 3,3'-diindole allyl acetate
[0043]
[0044] In a 25mL flask, dissolve 221mg (1mmol) (4-methoxyphenyl) allyl aminoacetate in 5.0mL acetonitrile, then add 246mg (2.1mmol) indole, 4.8mg (1mol%) rhodamine 6G , 315mg (1.5eq) of citric acid, stirred at room temperature for 12 hours under sunlight irradiation. Then add saturated sodium bicarbonate solution to quench the reaction, extract with ethyl acetate, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, column chromatography (petroleum ether / ethyl acetate=4:1) to obtain 307mg product, yield 93 %. red solid. 1 H NMR (400MHz, CDCl 3 ): δ 7.86(s,2H),7.51(d,J=8.0Hz,2H),7.17-7.03(m,4H),6.81(s,2H),5.81(m,1H),5.44(s,1H ),5.17-5.04 (m,2H),4.55(m,2H). 13 C NMR (100MHz, CDCl 3 )δ172.7, 136.9, 132.7, 126.8, 123.7, 121.3, 119.0, 118.8, 117.1, 113.1, 111.4, 64.8, 40.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com