A kind of preparation method of cefotaxime sodium
A technology of cefotaxime sodium and cefotaxime acid is applied in the field of preparation of antibacterial drug cefotaxime sodium, can solve problems such as unfavorable production, research and utilization, unprotected functional groups, easy oxidative degradation and the like, and achieves improvement of market competition Good strength and color grade, reducing the effect of degradation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0068]Add 500ml of chloroform, 20g of 7-aminocephalosporanic acid and 22g of HMDS at room temperature. Increase the temperature and reflux for 5-6.0hr, then lower the temperature to below 20°C and add 28g of AE-active ester for condensation for 12-15hr.
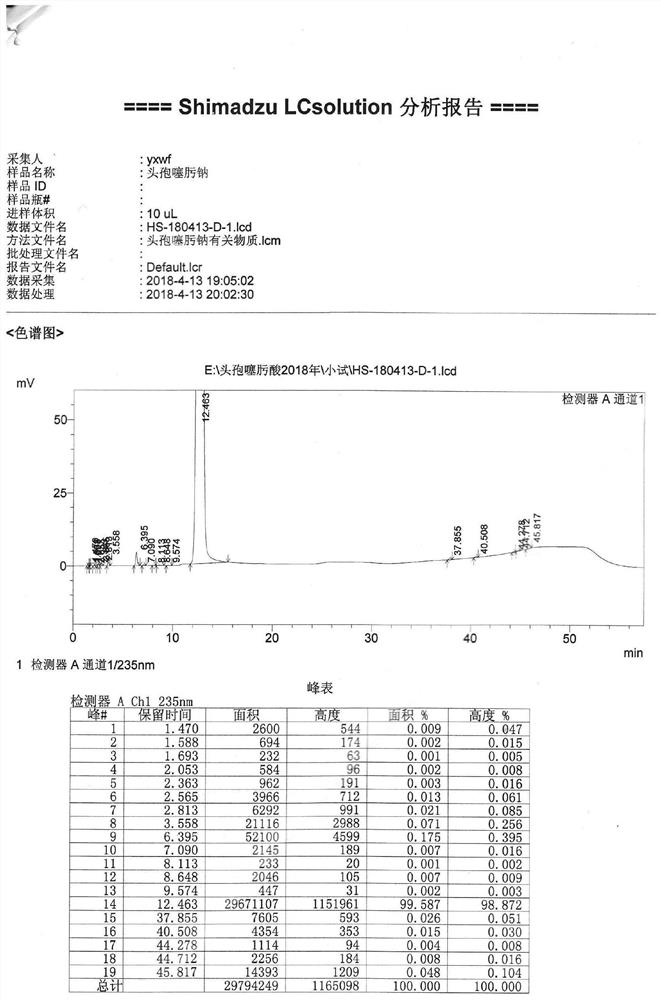
[0069]Then, 300 ml of 8% sodium bicarbonate aqueous solution was added for extraction, and 2 g of activated carbon was added to the water phase for decolorization for 20 minutes and filtered. The temperature of the filtrate was controlled below 30°C, 130ml of acetone and 65ml of n-butanol were added, and the dilute acid solution was added dropwise to adjust the pH to 2.8-3.0, and crystals were precipitated. After filtering, the filter cake is washed with pure water, drained and then washed with acetone, drained and dried to obtain a crude cefotaxime acid with a yield of 85%.
[0070]The purity of the high-pressure liquid phase is 99.6%, and the color is yellow-green ≤2#. (See attachedfigure 1 )
Embodiment 1-2
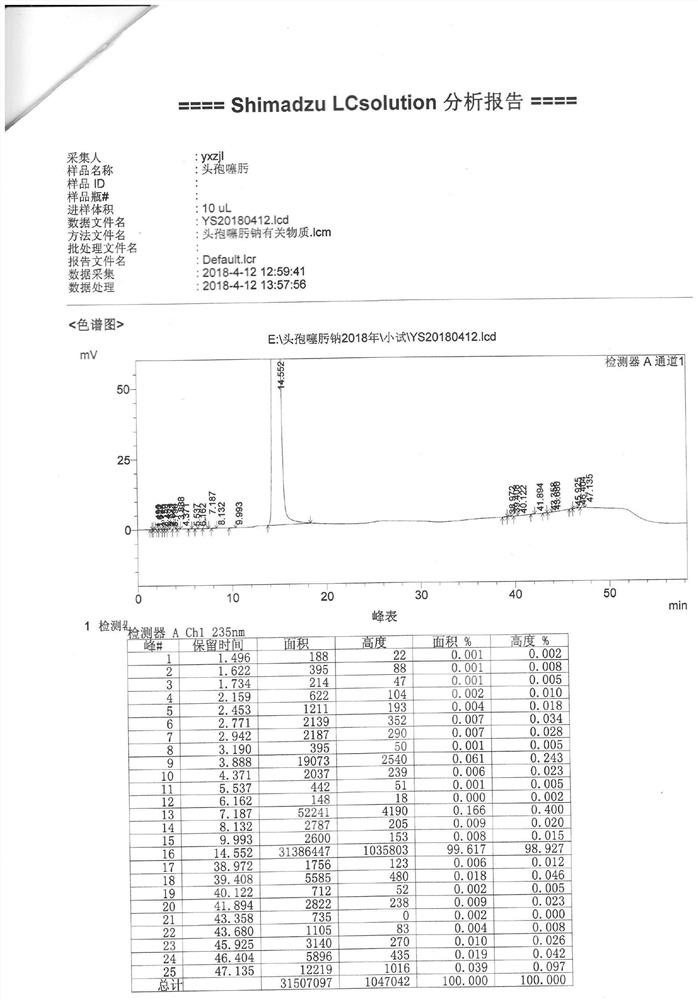
[0072]Add 300 ml of anhydrous methanol to the reactor, add 15 g of sodium acetate, and stir to dissolve. Then add 45 g of crude cefotaxime acid, stir to dissolve. Add 2g of activated carbon, decolorize for 20min, and filter. The filtrate was dripped with acetone until it became turbid. Cultivate crystals for 60 minutes. Then add dropwise acetone in the later stage, lower the temperature to 0-5°C, and grow the crystals for 60 minutes. Suction filtration and drying gave cefotaxime sodium with a yield of 87%.
[0073]The purity of the high-pressure liquid phase is 99.6%, and the color is yellow-green ≤2#. (See attachedfigure 2 )
Embodiment 2-1
[0075]Add 300ml of dichloromethane, 20g of 7-aminocephalosporanic acid and 35g of BSU at room temperature. Increase the temperature and reflux for 5-6.0hr, then lower the temperature to below 20°C and add 30g of AE-active ester for condensation for 12-15hr.
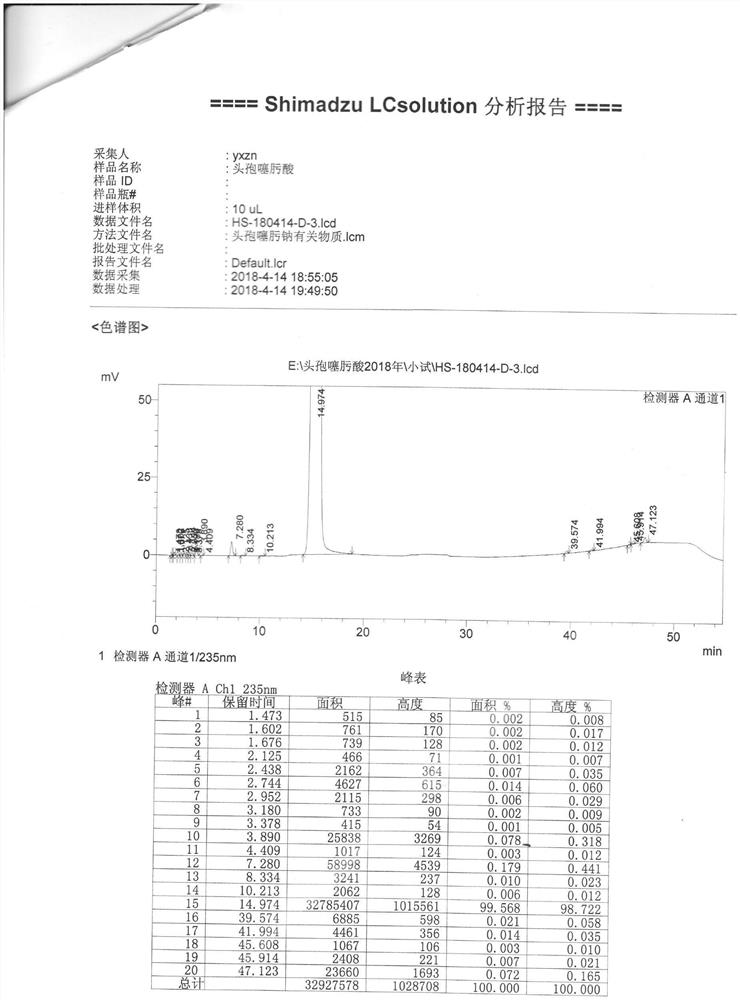
[0076]Then, 200ml of 6% sodium hydroxide aqueous solution was added for extraction, and 2g of activated carbon was added to the water phase for decolorization for 20 minutes and filtered. The temperature of the filtrate was controlled below 30°C, 130ml of tetrahydrofuran and 65ml of n-butanol were added, and a dilute acid solution was added dropwise to adjust the pH to 2.8-3.0, and crystals were precipitated. After filtering, the filter cake was washed with pure water, drained and then washed with tetrahydrofuran, drained and dried to obtain crude cefotaxime acid with a yield of 88%.
[0077]The purity of the high-pressure liquid phase is 99.6%, and the color is yellow-green ≤2#. (See attachedimage 3 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com