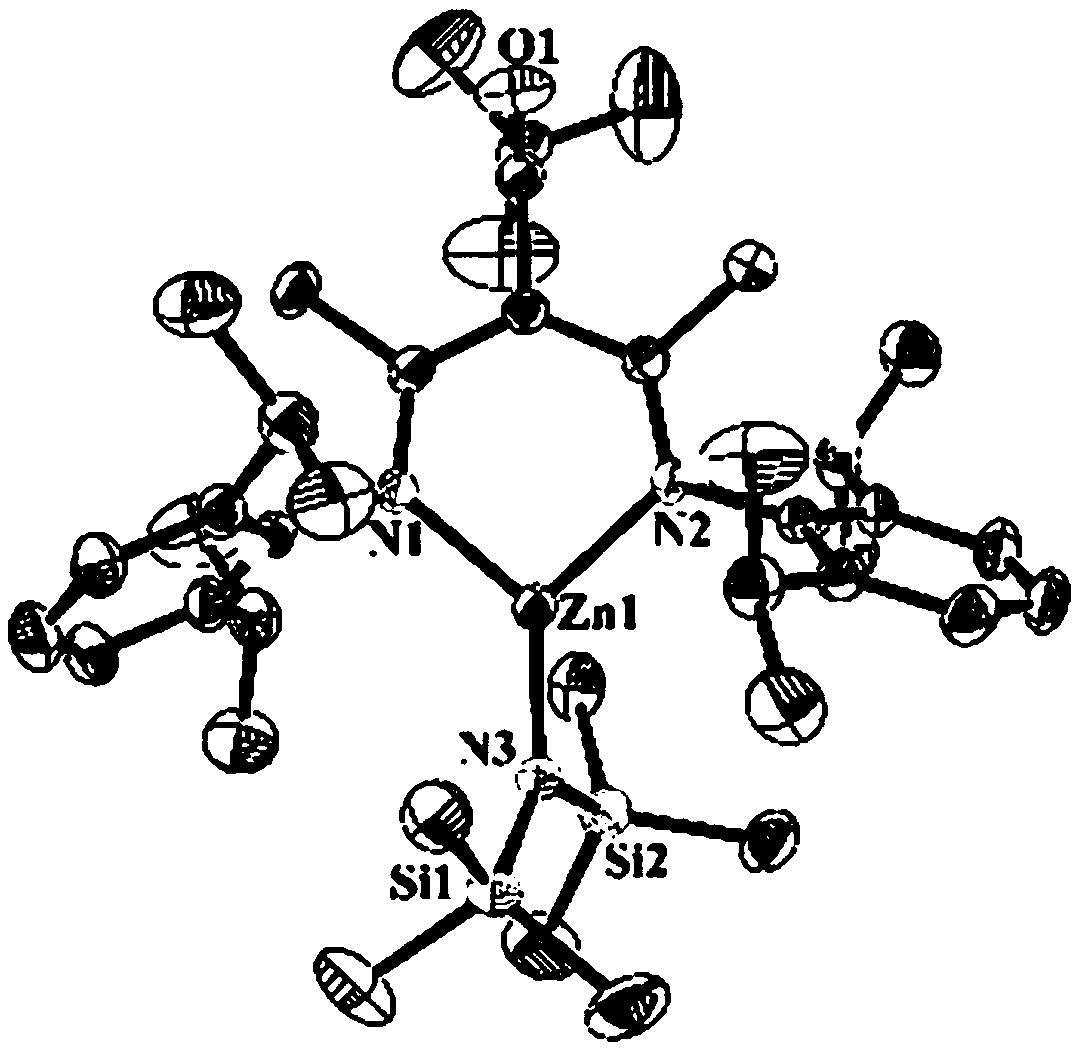
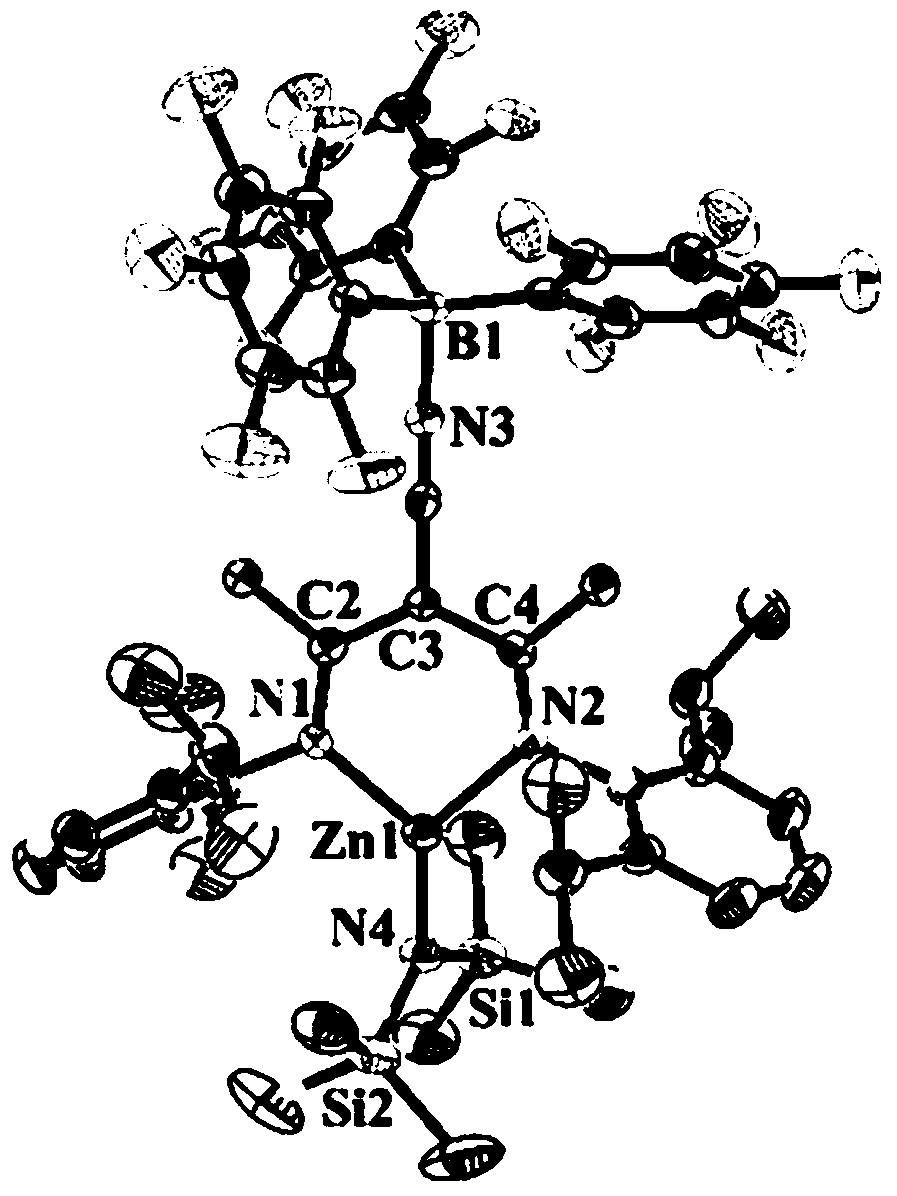
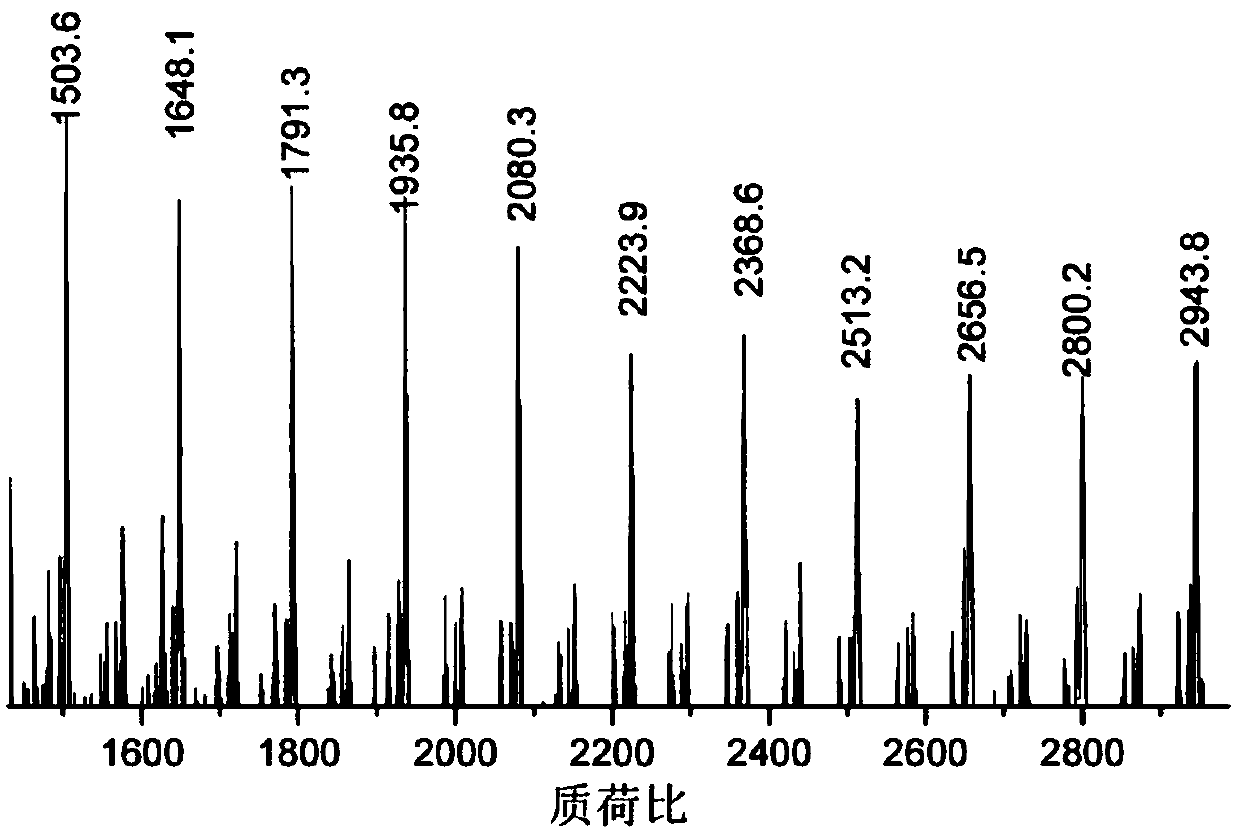
Beta-diimine zinc catalyst, and ligand, preparation method and application thereof
A technology of zinc complexes and ligand compounds, applied in the field of preparation, β-diimine zinc catalysts and their ligands, can solve the problems of reduced electron density of catalytically active metal centers, inability to regulate catalytic activity, etc., and achieve stereoselective sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0061] The following examples illustrate different aspects of the invention and present data including the synthesis of ligands, the synthesis of metal complexes and their application to the ring-opening polymerization of lactide and caprolactone, wherein the synthesis of metal complexes, ring-opening The polymerization process is carried out under anhydrous and oxygen-free conditions. All sensitive substances are stored in a glove box refrigerator at -30°C. All solvents are strictly dried to remove water. Lactide is purified by recrystallization from dichloromethane and n-hexane. Caprolactone was obtained by drying calcium hydride for 12 hours and then purifying under reduced pressure. The zinc precursor zinc compound of bis(bistrimethylsilyl)amine was obtained according to the literature [Rivillo D, Gulyás H, Benet-Buchholz J, et al.Angewandte Chemie International Edition, 2007, 46(38): 7247-7250.] The synthesis was obtained, without special instructions, all the raw material...
Synthetic example 1
[0064] Synthesis Example 1: Synthesis of 2-((2,6-diisopropyl)amino)-3-cyano-4-((2,6-diisopropylphenyl)imino)-2-pentene
[0065]
[0066] Under nitrogen protection, add 2-((2,6-diisopropyl) amino)-4-((2,6-diisopropylphenyl) imino)-2-pentene ( 1.8 g, 4.3 mmol), and then 90 ml of tetrahydrofuran was added. After cooling the mixture solution to -78°C by liquid nitrogen / acetone, slowly add n-butyllithium (2.5 mol / L, 5.16 mmol, 2.06 ml) to the reaction system, react at -78°C for 5 minutes, and then Warm to room temperature for another 1 hour. After cooling down to -78°C again, p-toluenesulfonyl cyanide (0.82 g, 4.52 mmol) dissolved in 30 ml of tetrahydrofuran was added dropwise to the reaction system, and then warmed to room temperature for 10 hours. After the reaction is over, use a rotary evaporator to spin off the solvent, then dissolve the obtained solid with dichloromethane, and then extract the organic phase three times with saturated aqueous sodium chloride solution. After...
Synthetic example 2
[0071] Synthesis Example 2: 2-((2,6-diisopropyl)amino)-3-tert-butyryl-4-((2,6-diisopropylphenyl)imino)-2-pentene synthesis
[0072]
[0073] Under nitrogen protection, add 2-((2,6-diisopropyl) amino)-4-((2,6-diisopropylphenyl) imino)-2-pentene ( 4.18 g, 10 mmol), and then 100 ml of tetrahydrofuran was added. After cooling down to -78°C with liquid nitrogen / acetone, slowly add n-butyllithium (2.5 mol / L, 11.2 mmol, 4.5 ml) into the reaction system, react at -78°C for 5 minutes, then warm the reaction Allow to react for another 1 hour at room temperature. After cooling down to -78°C again, trimethylacetyl chloride (1.3 mL, 10.5 mmol) was added dropwise to the reaction system, and then warmed to room temperature for 12 hours. After the reaction was finished, spin off the solvent with a rotary evaporator, dissolve the solid with dichloromethane, and then extract three times with saturated aqueous sodium chloride solution. After the extraction, separate the organic phase with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com