Dinuclear half-sandwich iridium complex containing bisimine ligand and preparation method and application thereof
A technology of iridium complex and bis-imine, applied in the field of synthetic chemistry, can solve problems such as product yield decline, and achieve the effects of less by-products, high thermal stability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of the binuclear semi-sandwich iridium complex containing double imine ligand
[0023]
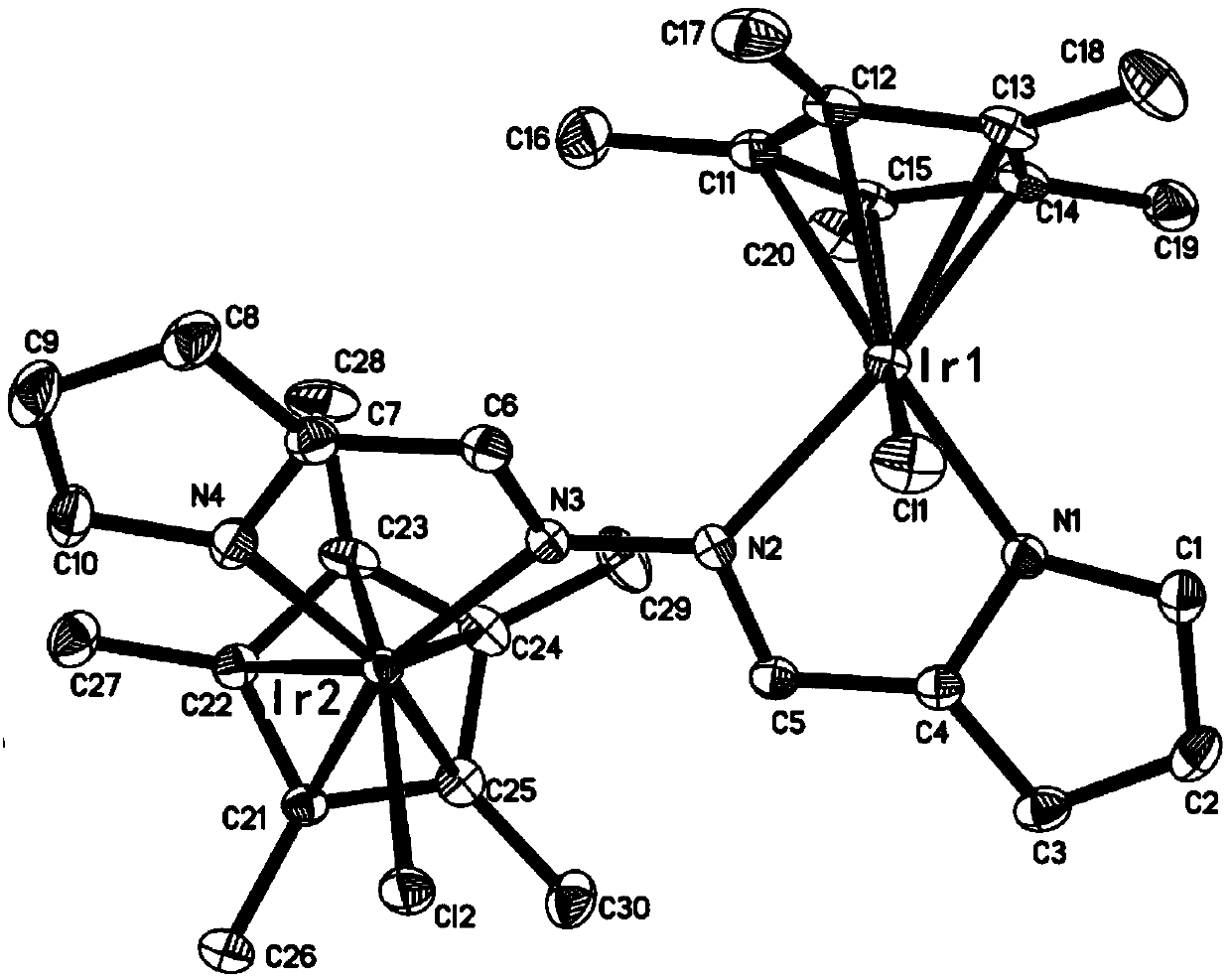
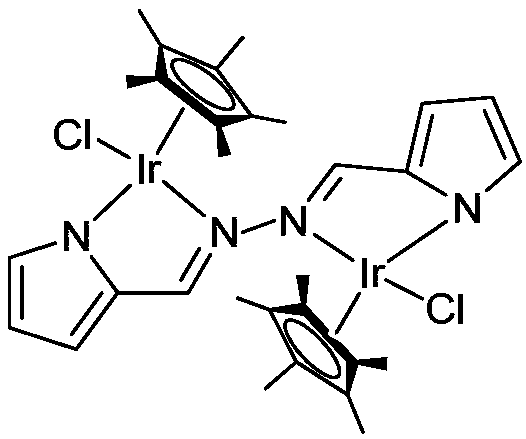
[0024] At -78°C, n-BuLi (1.6M) in n-hexane (1.0 mL, 1.6 mmol) was added dropwise to the pyrrole-cyclobis-imine ligand C 10 h 10 N 4 (119.0mg, 0.64mmol) in tetrahydrofuran solution, continue to stir for 50 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 60 minutes, then add pentamethylcyclopentadienyl iridium chloride dimer [Cp * IrCl 2 ] 2 (460.0mg, 0.58mmol), continued to react at room temperature for 4 hours; after the reaction was over, it was left to stand and filtered, and the solvent was drained under reduced pressure, and the crude product obtained was separated by column chromatography (petroleum ether / dichloromethane (v / v )=6:1) to obtain orange-red target product C 30 h 38 Cl 2 Ir 2 N 4 (437.0 mg, 83% yield). Its crystal structure is shown in figure 1 shown. Single crystal culture met...
Embodiment 2
[0027] Example 2: Oxidation of halogenated hydrocarbons to aldehydes catalyzed by dinuclear semi-sandwich iridium complexes containing bis-imine ligands
[0028]
[0029] The catalyst prepared in Example 1 was used to catalyze the oxidation reaction of halogenated hydrocarbons: to bromoethane (1mmol, 109mg) and DMSO solution containing binuclear semi-sandwich iridium complex (0.001mmol, 0.9mg) was added, open reaction, reaction The temperature is 30°C, and the reaction time is 270 minutes. After the reaction, the reaction solution is washed with water, extracted and concentrated. The crude product is separated by silica gel column chromatography and dried until the quality remains unchanged to obtain the corresponding aldehyde compound C 2 h 4 O (41 mg, 93% yield), elemental analysis: C 54.53, H 9.15 (theoretical); C 54.43, H 9.19 (actual).
Embodiment 3
[0030] Example 3: Oxidation of halogenated hydrocarbons to aldehydes catalyzed by dinuclear semi-sandwich iridium complexes containing bis-imine ligands
[0031]
[0032] Adopt the catalyst prepared in Example 1 to catalyze the oxidation reaction of halogenated hydrocarbons: add the DMSO solution containing binuclear half-sandwich iridium complex (0.001mmol, 0.9mg) to chloropropane (1mmol, 80mg), open reaction, reaction temperature 50°C, the reaction time is 300 minutes. After the reaction, the reaction solution is washed with water, extracted and concentrated. The crude product is separated by silica gel column chromatography and dried until the quality remains unchanged to obtain the corresponding aldehyde compound C 3 h 6 O (55 mg, 95% yield), elemental analysis: C 62.04, H 10.41 (theoretical); C 62.03, H 10.53 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com