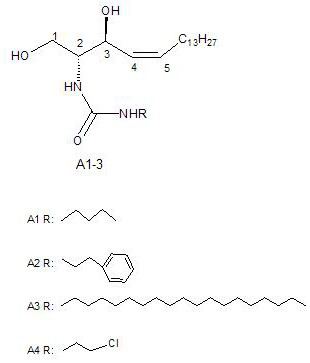
Ceramide analog a and its preparation method and application
A technology for ceramides and analogs, applied in the field of medicine, can solve the problems of biological application obstacles, bottlenecks in the chemical synthesis of ceramides, and the activity needs to be improved, and achieves the effects of improving solubility, inhibiting tumor cell proliferation, and being easy to control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Neuronamide analog A synthesis
[0034] 1) 4,5 - Construction of cis spanner
[0035] The raw material uses a contained galactose as a chiral source. The first step is a 4,6-bit hydroxyl group of galactose; the second step is oxidized, and the aldehyde group is generated at a 3-gyroxyl group, and the sugar 1, 2 bits of carbon; the third step is used with wittg reaction, and Pre-synthesized fourteen carbon phosphorid reacted, 4,5-cisole olefin, which is a relatively key step; the fourth step is supernatified in carbon on the original 5-position hydroxyl group, in this position and carbon-structured Turning to obtain the desired structure; fifth step descending ketone reaction; the sixth stepped nitrogen is converted to an amino group, obtained (2S, 3R, 4Z) -2-amino-1,3-octadecyl diol (4, 5-cis spanner B). (4,5-cis sphingol A) See CN201410678188.5)
[0036] 2) 1 grams of 4,5-cis sphingol A is dissolved in 30 ml of chloroform, 40 ° C is dissolved, after lowering to room temper...
Embodiment 2
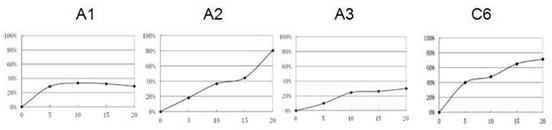
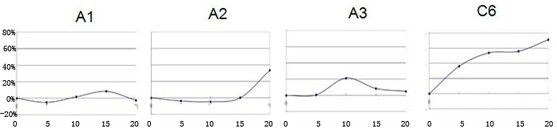
[0050] Neuronamide analog A inhibits human colorectal cancer cells, human pancreatic cancer cell proliferation.
[0051] Biological experiments are employed in human cancer cells, respectively, colorect cancer cells LS174T, colon cancer cell SW480, colon cancer cell SW620; in situ pancreatic adenocarcinoma cell BXPC-3, pancreatic cancer cells PANC-1, pancreatic cancer cells SW1990. The culture system is DMEM culture solution, which contains 10% fetal bovine serum, then placed at 37 ° C, 5% CO 2 , Cultured under saturation humidity. The new neuramide analogs A (A1, A2, A3) and the control C6 (purchase) were formulated into a culture system, and 10% fetal bovine serum was included, and then placed at 37 ° C, 5% CO. 2 , Cultured under saturation humidity. The new neural amide analog A and the control C6 (purchase) were formulated into 5 μm, 10 μm, 15 μm, 20 μm, and all samples were fully dissolved.
[0052] (1) Select the tumor cells in which the long-term long-term tumor cells are m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com