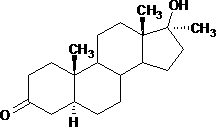
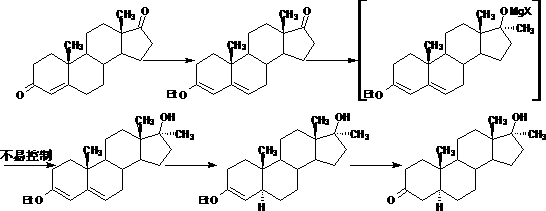
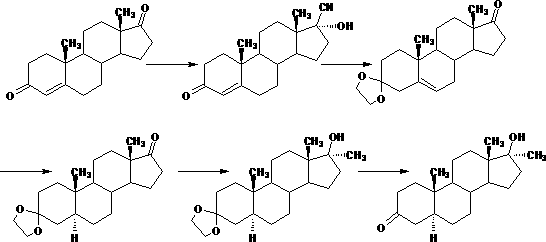
Preparation method of androstane-17alpha-methyl-17beta-hydroxy-3-ketone
A technology of methyl and hydroxyl, applied in the field of organic chemical synthesis, can solve the problems of similar polarity of products, affecting product purity, difficult to remove, etc., achieve low production cost, easy control of production process, and ensure the effect of yield level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Preparation of compound 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal
[0034] Add 20g of methyltestosterone, 100ml of dichloromethane, 30ml of triethyl orthoformate, 40ml of ethylene glycol and 1ml of boron trifluoride ether into the reaction bottle, stir well and keep warm at 30°C to 35°C for 10 hours to react; after the reaction is completed , lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml methanol, concentrate under reduced pressure to a paste, add 20ml methanol Reflux for 30 minutes; cool down to below 5°C, filter and dry to obtain 22.1g of 5-androstene-17α-methyl-17β-hydroxy-3-ethylene glycol ketal;
[0035] 2. Preparation of the compound androst-17α-methyl-17β-hydroxyl-3-one
[0036]Add 20g of 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal, 320ml of methanol and 80ml of dichlor...
Embodiment 2
[0039] 1. Preparation of compound 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal
[0040] Add 20g of methyltestosterone, 80ml of chloroform, 40ml of triethyl orthoformate, 60ml of ethylene glycol and 2ml of boron trifluoride ether into the reaction bottle, stir well and keep warm at 30°C to 35°C for 9 hours to react; after the reaction is completed , lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml methanol, concentrate under reduced pressure to a paste, add 20ml methanol Reflux for 30 minutes; cool down to below 5°C, filter and dry to obtain 22.3g of 5-androstene-17α-methyl-17β-hydroxy-3-ethylene glycol ketal;
[0041] 2. Preparation of the compound androst-17α-methyl-17β-hydroxyl-3-one
[0042] Add 20g of 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal, 270ml of methanol and 90ml of dichlorometha...
Embodiment 3
[0045] 1. Preparation of compound 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal
[0046] Add 20g of methyltestosterone, 120ml of dichloromethane, 60ml of triethyl orthoformate, 60ml of ethylene glycol and 1ml of boron trifluoride ether into the reaction bottle, stir well and keep warm at 30°C to 35°C for 9 hours to react; , lower the temperature to below 5°C, add triethylamine dropwise to adjust the pH value to 7.0-7.5, and stir for 30 minutes after addition; evaporate all solvents under reduced pressure, entrain with 20ml methanol, concentrate under reduced pressure to a paste, add 20ml methanol Reflux for 30 minutes; cool down to below 5°C, filter and dry to obtain 22.0g of 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene glycol ketal;
[0047] 2. Preparation of the compound androst-17α-methyl-17β-hydroxyl-3-one
[0048] Add 20g of 5-androstene-17α-methyl-17β-hydroxyl-3-ethylene ketal, 200ml of methanol and 100ml of dichloromethane into the reaction flask, sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com