Red organic coloring agent applied to resin coloring and preparation method thereof
An organic colorant and organic solvent technology, applied in the field of red organic colorant and its preparation, can solve the problems of no rigid structure, difficult to solve resin coloring, unable to form crystallinity, etc. The effect of good temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
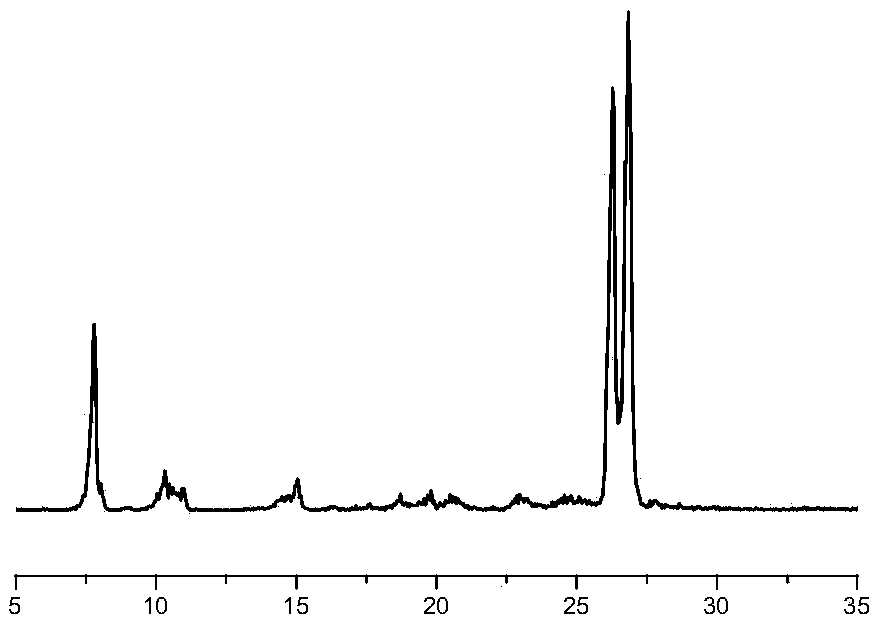
Image
Examples
Embodiment 1
[0057] The reaction formula is:
[0058]
[0059] Wherein: X is selected from Br; R is selected from -H.
[0060] In a 250 mL four-necked flask, 7.1 parts of 6-bromo-N-methyl-anthrapyridone, 1.87 parts of benzomelamine, 0.42 parts of cuprous iodide and 90 mL of alkylbenzene were added. Raise the temperature to 170°C, keep the reaction for 30 minutes, then gradually add 2.1 parts of sodium bicarbonate in portions to adjust the pH of the reaction, and react at a temperature of 165°C to 170°C for 12 hours. The complete disappearance of the reactant was traced by liquid chromatography, and the temperature was lowered to 90-95°C. Filter to recover the alkylbenzene mother liquor. Wash the filter cake with a small amount of ethanol until the filtrate is colorless, and recover the washing liquid. Wash the filter cake with water until the pH is neutral, and move to the next step.
[0061] Take the filter cake and add 100 ml of butanol solution, stir well for 2 hours, then raise ...
Embodiment 2
[0063] The difference from Example 1 is that in the above preparation process, cuprous chloride was used instead of cuprous iodide as a catalyst, and the operation steps of Example 1 were repeated to obtain about 5.9 grams of dry powder. Appearance: Brilliant red powder, yield 83.7%.
Embodiment 3
[0065] The difference from Example 1 is that o-dichlorobenzene is used instead of alkylbenzene as a solvent, and in a 250mL four-necked flask, 6.8 parts of 6-bromo-N-methyl-anthrapyridone, 1.87 parts of benzomelamine, Add 0.42 parts of catalyst cuprous iodide, add 80 ml of o-dichlorobenzene solution, raise the temperature to 170 ° C, keep warm for reaction, add 2.1 parts of acid-binding agent sodium bicarbonate in stages, adjust the pH of the reaction, react for 12 hours, and perform liquid chromatography It shows that the consumption of raw materials in the system is complete, and the reaction ends. Cool down to 90-95°C, filter, wash with o-dichlorobenzene until the filtrate is basically colorless, then wash with alcohol and water until neutral to obtain a wet filter cake, and recover the filtrate.
[0066] Take the filter cake and add 100 ml of butanol solution, fully stir for 2 hours, heat up to reflux, keep warm and reflux for 3 hours, cool down and filter, wash with water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com