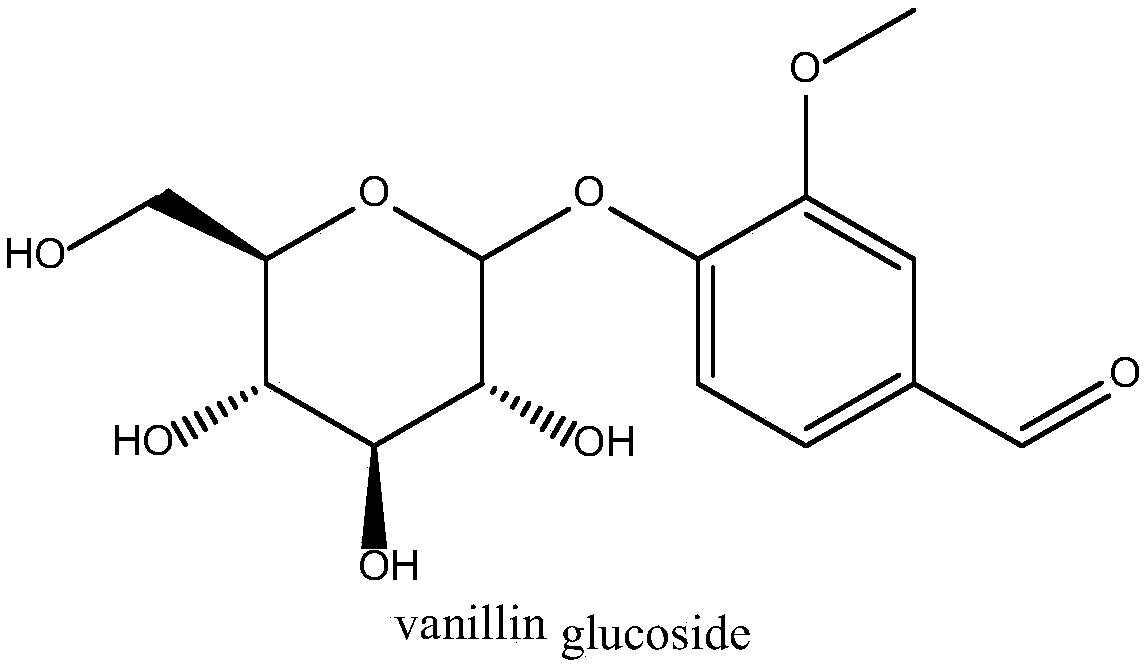
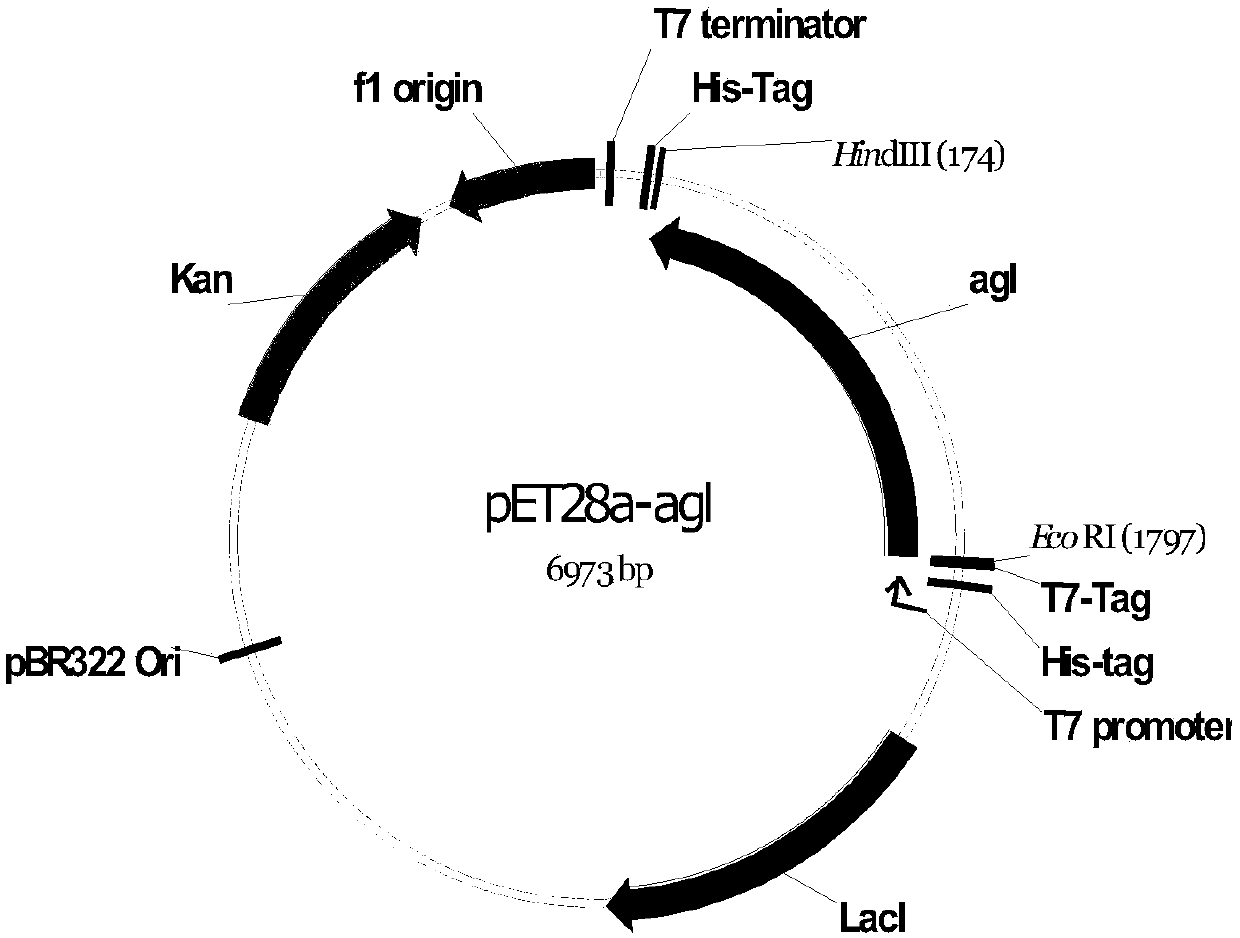
Glucosyltransferase and application thereof in producing vanillin-alpha-D-glucoside
A technology of glucosyl and glucoside, applied in the field of glucosyl transferase and its production of vanillin-α-D-glucoside, can solve problems such as premature release of flavor, and achieve the effects of high conversion rate and high product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, preparation produces the bacterial agent of vanillin-alpha-D-glucoside
[0023] 1. Extraction of Xanthomonas campestris genomic DNA
[0024] Xanthomonas campestrispv. campestris (Xanthomonas campestrispv. campestris) CGMCC No.13990 (disclosed in patent application CN107446844A) was activated by streaking on NYGA medium, cultured at 28°C for 36h, and a single colony was picked on NYGB medium in 30°C and 180rpm for 24 hours; use a 2mL centrifuge tube to take 1.5-3mL of bacterial liquid, centrifuge at 10000rpm for 2min, discard the supernatant, and wash the bacterial cells with deionized water for 2-3 times; 600 μL of cell lysate (40mM Tris-acetic acid buffer pH 7.8, 20mM sodium acetate, 1mM EDTA, 1% SDS) was pipetted sufficiently, and placed in ice water for 5min; then added 200μL of 5mol Invert / L NaCl back and forth 8-10 times, a white precipitate forms; to remove impurities and cell debris, centrifuge at 12,000 rpm at 4°C for 10 min; after centrifugation...
Embodiment 2
[0037] The application of embodiment 2 bacterial agents in the production of vanillin-α-D-glucoside
[0038] 1. Activity detection of bacterial agents
[0039] Get 10 mL of the bacterial agent cultured in Example 1, collect the cells by centrifugation at 5000 × g, resuspend 0.3 g of the cells in 10 mL of 50 mM phosphate buffer at pH 7.0; add vanillin and final concentration of 7.6 g / L Maltose with a concentration of 400g / L was catalyzed on a water bath shaker at 30°C and 150rpm for 30min. The reaction solution was used for HPLC analysis.
[0040] Liquid chromatography detection conditions. Sample pretreatment: Add 100 μL of reaction solution to 900 μL of 0.01mol / L dilute hydrochloric acid; centrifuge at 12000×g for 5 minutes, filter with a 0.22 μm filter membrane, and add the filtrate to a liquid phase vial; chromatographic column: C l8 Column, 250×4.6mm; column temperature: 25°C; mobile phase: CH 3 OH (methanol): H 2 O=35:65 (volume ratio); flow rate: 1.0mL min -1 ; Det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com