A solid dosage form of a prodrug derivative containing ubenimex and a preparation method thereof
The technology of a solid dosage form, ubenimex, is applied in the field of solid dosage forms of prodrug derivatives and its preparation, which can solve the problems of low bioavailability, large toxic and side effects, and poor fat solubility, etc., to prolong the residence time and improve biological Utilization, effect of improving pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] For the compound pentafluorouracil-ubenimex (5FU-Bestatin), the preparation method may include: reacting pentafluorouracil with triphosgene under the catalysis of activated carbon, and then reacting with ubenimex to obtain the target compound 5FU-Bestatin.
[0043] Its specific synthetic route is as follows:
[0044]
[0045] Reaction condition a: BTC, activated carbon, Py, 0°C, where BTC is triphosgene, Py is pyridine
[0046] Concrete preparation steps are as follows:
[0047] Put 0.65g of pentafluorouracil (5mmol) in the reaction flask, add 20ml of pyridine to dissolve it completely, then add 0.65g of activated carbon, slowly add 0.5g of triphosgene under ice bath, continue to react under ice bath for 1 hour, draw out Phosgene, rushed into N 2 , for three times. Under ice-cooling, 1.7 g of ubenimex (5 mmol) was slowly added, followed by reaction at room temperature for 12 hours. Activated carbon was filtered, extracted with ethyl acetate, washed three times wi...
preparation example 1
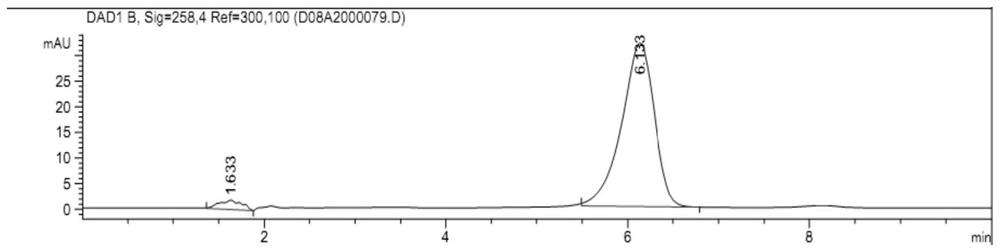
[0072] Put 0.65g of pentafluorouracil (5mmol) in the reaction flask, add 20ml of pyridine to dissolve it completely, then add 0.65g of activated carbon, slowly add 0.5g of triphosgene under ice bath, continue to react under ice bath for 1 hour, draw out Phosgene, rushed into N 2 , for three times. Under ice-cooling, 1.7 g of ubenimex (5 mmol) was slowly added, followed by reaction at room temperature for 12 hours. Activated carbon was filtered, extracted with ethyl acetate, washed three times with 6 mol / L hydrochloric acid solution, washed three times with saturated brine, and dried over anhydrous sodium sulfate. Ethyl acetate was distilled off until the precipitation of solids ceased, put into the refrigerator for crystallization, and filtered to obtain a white product, which was compound 5FU-Bestatin, a white solid, yield: 36.7%, melting point=165-167°C. MS-ESI: [M-1]=463.6; [M-1+Na]=485.8. Its proton nuclear magnetic resonance spectrum data are as follows:
[0073] 300M...
Embodiment 1
[0100]
[0101]
[0102] Compound 5FU-Bestatin, lactose monohydrate, microcrystalline cellulose, sodium carboxymethylcellulose and magnesium stearate were passed through a 100-mesh sieve. The sieved materials were placed in a three-dimensional mixer for total mixing, and the rotation speed of the three-dimensional mixer was 10 rpm for 15 minutes. The mixed materials were directly compressed into tablets or filled into capsules to prepare 100,000 units, each unit containing 20 mg of the compound 5FU-Bestatin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com