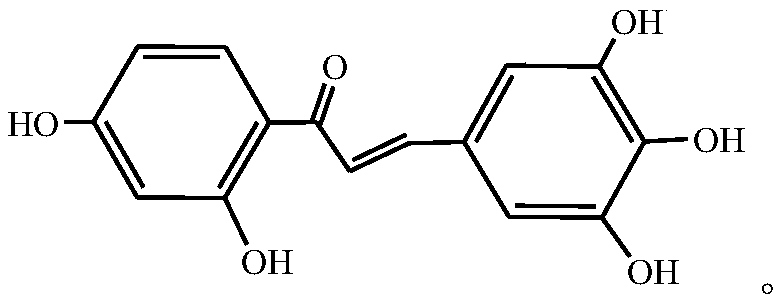
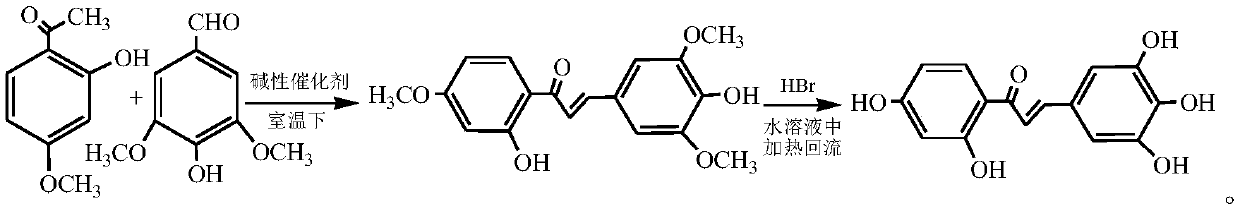
Synthesis method of Robtein
A synthesis method and alkaline technology, applied in the field of Robtein synthesis, can solve the problems of difficult purification, high production cost, single source, etc., and achieve the effects of time-saving and labor-saving synthesis process, short reaction time and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of basic catalyst
[0031] Weigh 30 parts of sodium hydroxide by weight, dissolve it in distilled water, then weigh 70 parts of aluminum oxide, add aluminum oxide to the above sodium hydroxide solution, stir evenly, evaporate the water under reduced pressure to dryness, and put the The obtained powder was dried in a drying oven at 105 °C for 3 hours, then placed in a muffle furnace for calcination at 600 °C for 5 hours, taken out from the muffle furnace and cooled in a desiccator to obtain a sodium hydroxide / alumina basic catalyst ,spare.
[0032] (2) Synthesis of Robtein
[0033] Weigh 3.5kg of syringaldehyde, dissolve with 88.5L of absolute ethanol, for subsequent use;
[0034] Weigh 3.2 kg of paeonol, dissolve it in 123 L of dehydrated alcohol in the reaction kettle; add 1.11 kg of standby alkaline catalyst at room temperature of 5 ° C, stir evenly, under continuous stirring, slowly add standby cloves in 35min Aldehyde ethanol solution; the reactio...
Embodiment 2
[0036] (1) Preparation of basic catalyst
[0037] Same as Example 1;
[0038] (2) Synthesis of Robtein
[0039] Weigh 6.5kg of syringaldehyde, dissolve it with 150L of absolute ethanol, for subsequent use;
[0040] Weigh 5.93kg of paeonol, dissolve with 200L of dehydrated alcohol in the reaction kettle; add 1.8kg of standby alkaline catalyst at room temperature of 18°C, stir evenly, under continuous stirring, slowly add standby cloves in 33min Aldehyde ethanol solution; the reaction solution gradually turned into a brownish-yellow turbid solution, and after continuing to stir for 13h, the reaction solution became turbid and brown, and the degree of reaction was detected by TLC method, and then stirred for 3h to ensure a sufficient reaction; after the reaction was completed, Add absolute ethanol to dissolve the organic matter as much as possible, quickly suction filtration to remove the basic catalyst; distill the suction filtrate under reduced pressure to recover ethanol, an...
Embodiment 3
[0042] (1) Preparation of basic catalyst
[0043] Same as Example 1;
[0044] (2) Synthesis of Robtein
[0045] Weigh 9.1kg of syringaldehyde, dissolve with 180L of absolute ethanol, for subsequent use;
[0046]Weigh 8.3 kg of paeonol, dissolve it in 260 L of absolute ethanol in the reaction kettle; add 2.3 kg of standby alkaline catalyst at room temperature of 26 ° C, stir evenly, under continuous stirring, slowly add standby cloves in 30 min Aldehyde ethanol solution; the reaction solution gradually turned into a brownish-yellow turbid solution, and after continuing to stir for 13h, the reaction solution became turbid and tan, the degree of reaction progress was detected by TLC method, and then stirred for 2h to ensure a sufficient reaction; after the reaction was completed, Add anhydrous ethanol to dissolve the organic matter as much as possible, quickly suction filtration to remove the basic catalyst; distill the suction filtrate under reduced pressure to recover ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com