Method for directly producing 1,2,6-hexanetriol by performing hydrogenation on 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and hexanetriol, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of long cycle, low yield, many reaction steps, etc., and achieve low cost, The effect of high yield and mild catalytic reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
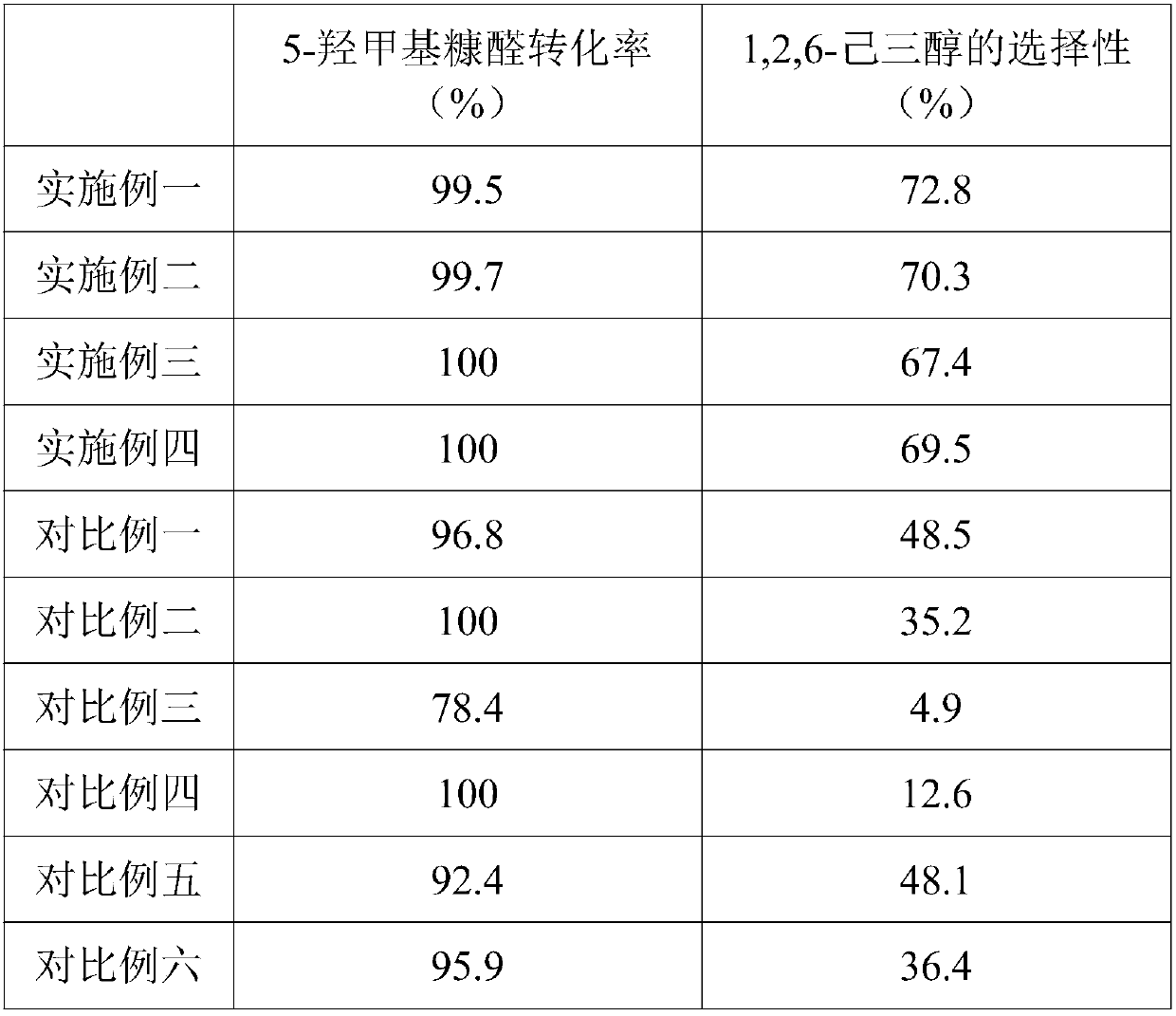
[0030] Example 1: A method for directly producing 1,2,6-hexanetriol by hydrogenation of 5-hydroxymethylfurfural
[0031] Its production method is: add 11.9g of cobalt chloride hexahydrate and 150mL of water into a 500mL round bottom flask, stir to dissolve and place it in an ice-water bath, and add 6.75g of KBH 4 Dissolve it in 150mL of water and place it in a dropping funnel. Under the protection of a nitrogen atmosphere, slowly add it dropwise to the above round bottom flask, the reaction proceeds rapidly, a large amount of gas is released, and Co-B precipitates are formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in absolute ethanol for later use. Put 0.2g of Co-B amorphous alloy catalyst, 0.5g of 5-hydroxymethylfurfural, and 10.0mL of ethanol into a 50mL stainless steel reactor with polytetrafluoroethylene, at a temperature of 140°C and a pressure of 1.5M...
Embodiment 2
[0032] Example 2: A method for directly producing 1,2,6-hexanetriol by hydrogenation of 5-hydroxymethylfurfural
[0033] Its production method is: add 5.95g of cobalt chloride hexahydrate, 5.95g of nickel chloride hexahydrate and 150mL of water into a 500mL round-bottomed flask, stir to dissolve and put it in an ice-water bath, and add 6.75g of KBH 4 Dissolve it in 150mL of water and place it in a dropping funnel. Under the protection of nitrogen atmosphere, slowly add it dropwise into the above round bottom flask, the reaction proceeds rapidly, a large amount of gas is released, and Co-Ni-B precipitates are formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in absolute ethanol for later use. Put 0.2g of Co-Ni-B amorphous alloy catalyst, 0.5g of 5-hydroxymethylfurfural, and 10.0mL of ethanol into a 50mL stainless steel reactor with polytetrafluoroethylene, at a...
Embodiment 3
[0034] Example 3: A method for directly producing 1,2,6-hexanetriol by hydrogenation of 5-hydroxymethylfurfural
[0035] Its production method is as follows: add 11.9g of cobalt chloride hexahydrate and 150mL of water into a 500mL round bottom flask, stir to dissolve and put it in an ice-water bath, and add 4.73g of NaBH 4 and 2.75g NaH 2 PO 2 Dissolve it in 150mL of water and place it in a dropping funnel. Under the protection of a nitrogen atmosphere, slowly add it dropwise to the above round bottom flask. The reaction proceeds rapidly, a large amount of gas is released, and Co-P-B precipitates are formed. When no gas is generated, filter the solid product, wash the solid product with deionized water and absolute ethanol for 3-5 times, and finally store the sample in isopropanol for later use. Put 0.2g of Co-P-B amorphous alloy catalyst, 0.5g of 5-hydroxymethylfurfural, and 10.0mL of isopropanol into a 50mL stainless steel reactor with polytetrafluoroethylene, at a tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com