Orange red light thermal activated delay fluorescent material and organic electroluminescence device
A thermal activation delay and fluorescent material technology, applied in the field of organic electroluminescent materials, can solve the problems of expensive raw materials, many synthesis and preparation steps, and difficult synthesis and preparation, and achieve simple synthesis and purification processes, fewer synthesis and preparation steps, and stable luminescence good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The reaction formula is as follows:
[0038]
[0039] The specific reaction is as follows:
[0040] 1. Add 0.40g (2.14mmol) 4-bromobenzene-1,2-diamine and 0.65g (1.78mmol) 3,6-dibromo-9,10-phenanthrenequinone into a 150mL three-necked flask, then add 100mL without Use water and ethanol as a solvent, stir under the protection of nitrogen, heat to 100°C, condense and reflux for 2 hours, a large amount of solid precipitates, filter and recrystallize to obtain light yellow solid 3,6,11-tribromodibenzo[a,c] Phenazine, its productive rate is 95%;
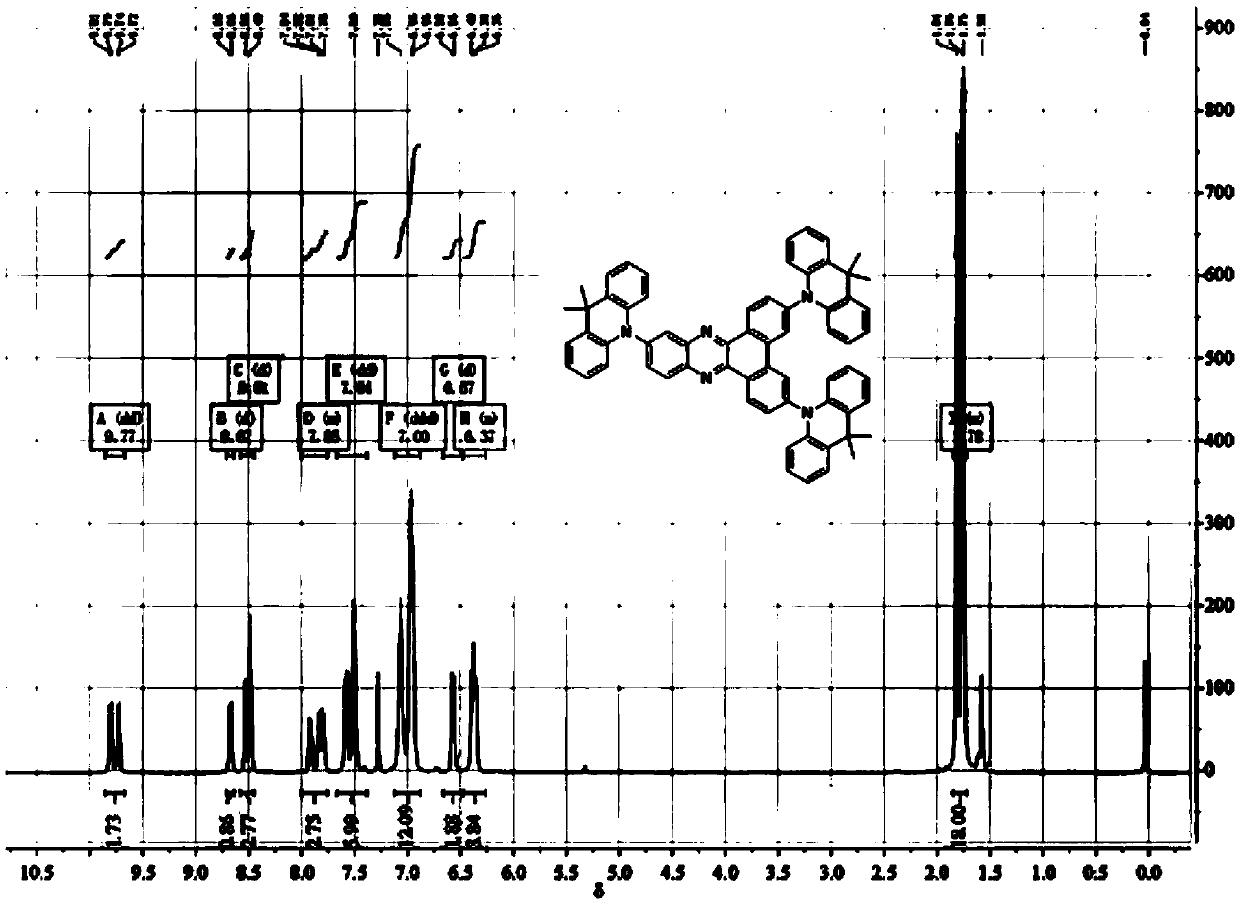
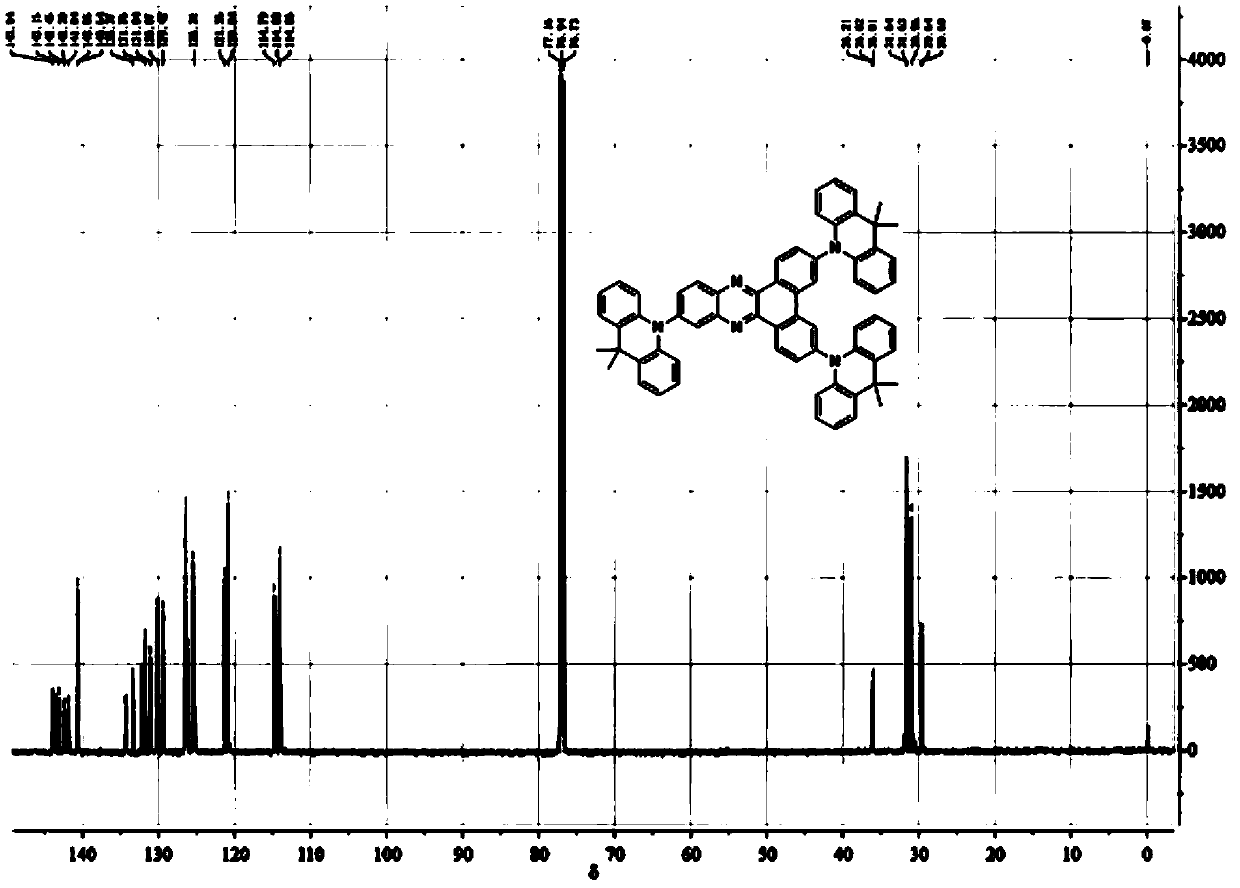
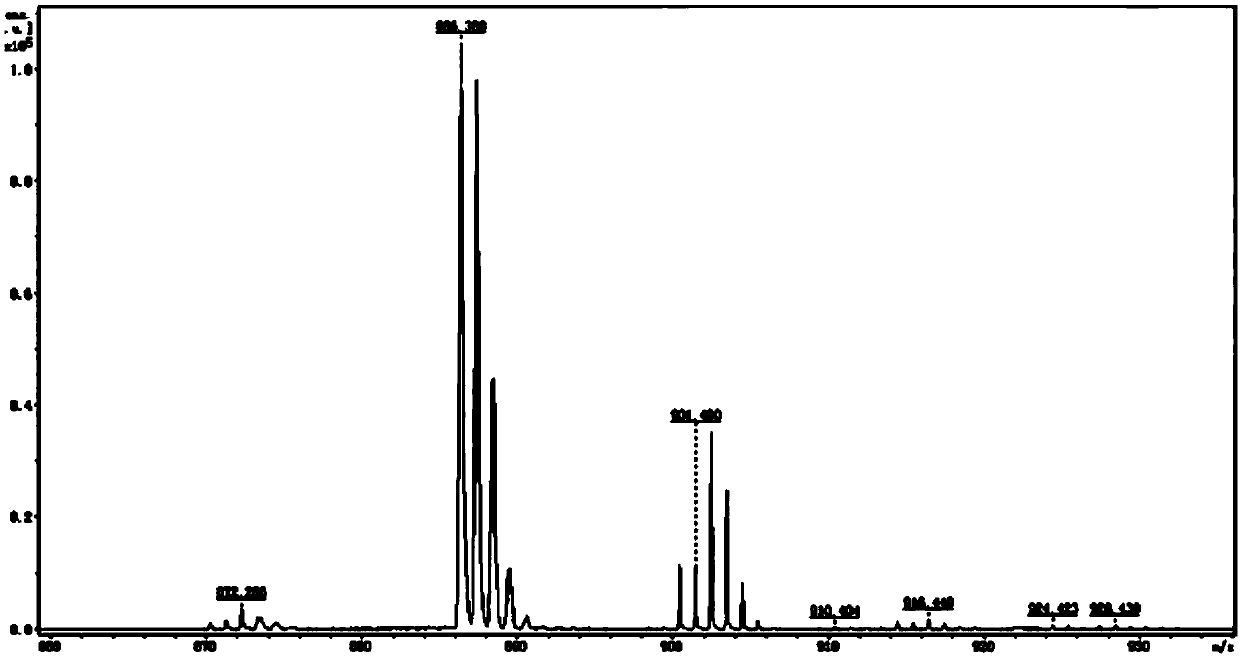
[0041] 2. Add 0.55g (1.06mmol) 3,6,11-tribromodibenzo[a,c]phenazine and 0.78g (3.72 mmol) 9,9-dimethyl-9,10 to a 150mL three-necked flask -Acridine, 50ml toluene, 0.41g (4.24mmol) sodium tert-butylate, 0.048g (0.053 mmol) tris(dibenzylideneacetone) dipalladium(0), 0.015g (0.053mmol) tetrafluoroboric acid Tri-tert-butylphosphine was heated to 110°C under nitrogen protection, and stirred and refluxed for 24h. After the reactio...
Embodiment 2
[0052] 3,6,11-tris(9,9-dimethylacridin-10(9H)-yl) dibenzo[a,c]phenazine (3DMAC-BP) prepared in Example 1 as the light-emitting layer Preparation and performance evaluation of organic electroluminescent devices.
[0053] Concrete preparation steps are as follows:
[0054] 1. The pretreatment of glass anode: choose the glass substrate that has 3 * 3mm indium tin oxide (ITO) film pattern as transparent electrode; After described glass substrate is washed with ethanol, process with UV-ozone again, obtain pretreatment Processed glass substrates.
[0055] 2. Vacuum evaporation: vacuum evaporation of each layer is carried out on the pretreated glass substrate by a vacuum evaporation method. First, put the processed glass substrate into the vacuum evaporation chamber, the vacuum degree is ≤2×10 -4 Pa, MoO 3 The deposition rate is The deposition rate of TADF luminescent material is about The deposition rate of the host material is about LiF layer deposition rate is Al depo...
Embodiment 3
[0061] 3,6,11-tris(9,9-dimethylacridin-10(9H)-yl) dibenzo[a,c]phenazine (3DMAC-BP) prepared in Example 1 as the light-emitting layer Preparation and performance evaluation of organic electroluminescent devices.
[0062] Concrete preparation steps:
[0063] Except for the doping concentration, the method for preparing an organic electroluminescent device is the same as in Example 2; The weight percentage of [a,c]phenazine in the light-emitting layer is 15%wt.
[0064] Performance evaluation:
[0065]The open-circuit voltage of the organic electroluminescence device made is 3.3V, and luminous wavelength is 600nm, and external quantum efficiency is 19.6%, and current efficiency is 37.9cd / A, and power efficiency is 35.0m / W, and CIE color coordinate value is (0.565 ,0.432).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com