Human glioma primary cells and in-vitro isolation and culture method and application thereof
A primary cell, isolation and culture technology, applied in the field of cell biology, can solve the problem that the real physiological response of clinical patients cannot be accurately reflected, the low-grade glioma-related research cannot provide an effective research system, and the loss of primary glioma cells can be solved. Heterogeneity and other issues to achieve the effect of stable traits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Primary isolation and culture of human high-grade glioma primary cells
[0039] (1) Through the hospital ethics committee, with the consent of the patient or the patient's guardian and after signing the informed consent, fresh clinical glioma resection specimens were obtained from Wuhan Union Medical College Hospital. The specimens were WHO grade glioblastoma.
[0040] (2) Immediately put the resected specimen into the pre-cooled sterile tissue preservation solution (containing 1000U / ml penicillin, 1000ug / ml streptomycin sulfate, 2.5ug / ml amphotericin and 50ug / ml gentamicin) Into the collection tube, and immediately put into a 4 ℃ sample transport box, transported to the laboratory within 4 hours for cell separation.
[0041] (3) Primary isolation culture: Obtain the tissue in a biological safety cabinet, rinse it once with absolute ethanol, and rinse it twice with 1xPBS (pH7.2-7.4), remove blood vessels, fat and For the necrotic tissue part, cut the tissue ...
Embodiment 2
[0043] Example 2: Primary isolation and culture of primary human low-grade glioma cells
[0044] (1) Through the hospital ethics committee, with the consent of the patient or the patient's guardian and signed the informed consent, a fresh clinical glioma resection specimen was obtained from Wuhan Union Medical College Hospital. The specimen was WHO grade II diffuse astrocytoma.
[0045] (2) Immediately put the resected specimen into the pre-cooled sterile tissue preservation solution (containing 1000U / ml penicillin, 1000ug / ml streptomycin sulfate, 2.5ug / ml amphotericin and 50ug / ml gentamicin) Into the collection tube, and immediately put into a 4 ℃ sample transport box, transported to the laboratory within 4 hours for cell separation.
[0046] (3) Primary isolation culture: Obtain the tissue in a biological safety cabinet, rinse it once with absolute ethanol, and rinse it twice with 1xPBS (pH7.2-7.4), remove blood vessels, fat and For the necrotic tissue part, cut the tissue ...
Embodiment 3
[0048] Example 3: Subculture of primary human glioma cells
[0049] (1) When the abundance of cells cultured in T25 culture flask reaches 80%, rinse the cells twice with 1xPBS (pH7.2-7.4), and add 1ml of 0.05% trypsin-EDTA to digest the monolayer cells for 2-3min.
[0050] (2) Add 2ml DF31 complete medium to stop the digestion.
[0051] (3) Centrifuge at 1000rpm for 4 minutes, remove the supernatant, collect the cell suspension, resuspend with 1ml DF31 complete medium, supplement the medium, and put it into a T25 culture bottle for cultivation according to the ratio of 1 to 2.
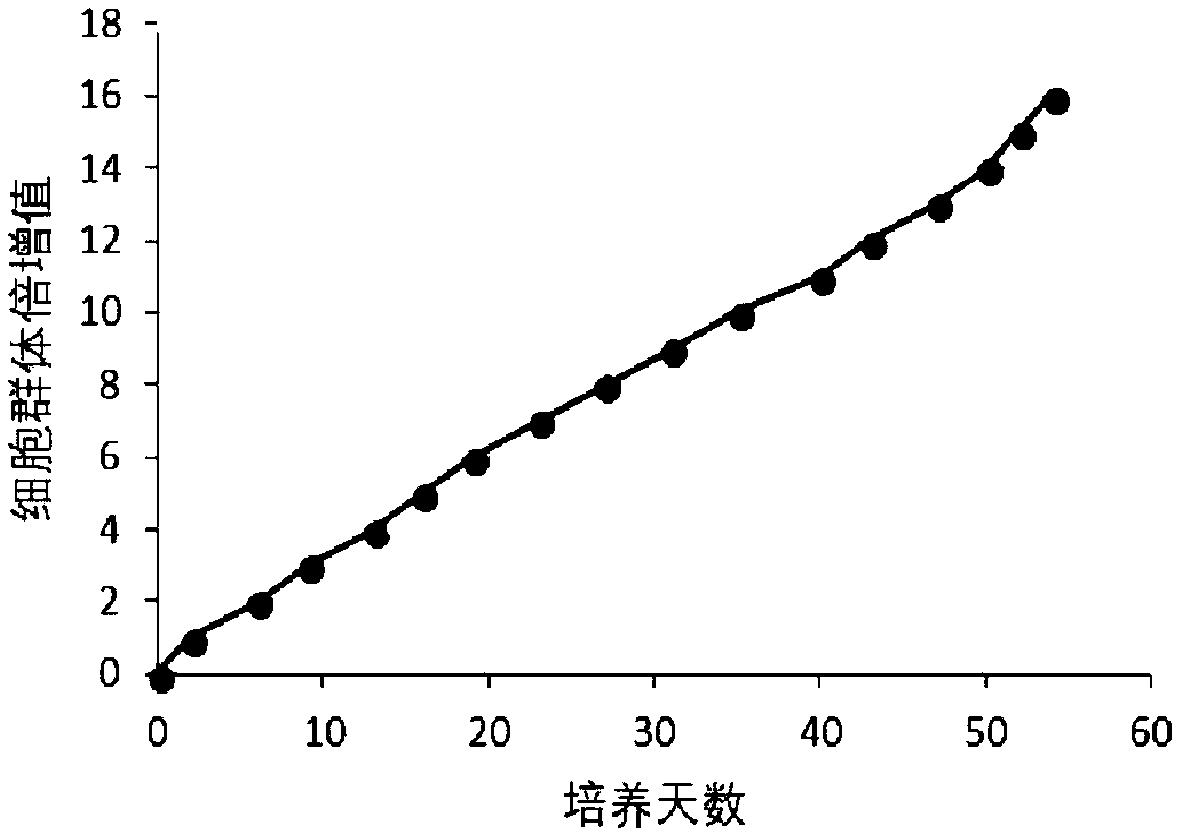
[0052] The human high-grade glioma primary cells and human low-grade glioma primary cells subcultured according to the above method, the cell growth curve of the culture establishment line is as follows: image 3 with Figure 4 , continuous passage for 50 days, the primary human glioma cells of the present invention can still maintain a proliferative state and grow normally.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com