Method for preparing mixed linear high value for centrifugal micro-fluidic chip
A microfluidic chip and centrifugal technology, which is applied in the field of medical testing, can solve the problem of not being able to evaluate multiple items at the same time, and achieve the effects of reducing testing costs, reducing matrix effects, and saving testing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Serum collected for physical examination, hepatitis B surface antibody, hepatitis A antibody, hepatitis C antibody, HIV antibody, etc. were all negative, and the serum was divided into two (equal volumes);
[0028] (2) Add 0.80% cholesteryl sulfate sodium salt to one of the serums, heat to 55°C, and cool to room temperature after completely dissolving;
[0029] (3) Add 0.25% urea, 0.025% creatinine, 0.035% uric acid, 0.85% glucose, 0.025% glycocholic acid, and 0.08% triolein to another serum in sequence;
[0030] (4), step (2) and step (3) gained solution are mixed;
[0031] (5), in the solution of step (4) gained, add the bovine serum albumin of 4.5%, the alanine aminotransferase of 500U / L, the aspartate aminotransferase of 500U / L;
[0032] (6) Add 5.0% mannitol and 0.1% sodium azide to the solution obtained in step (5); after fully dissolving and mixing, subpackage and freeze-dry.
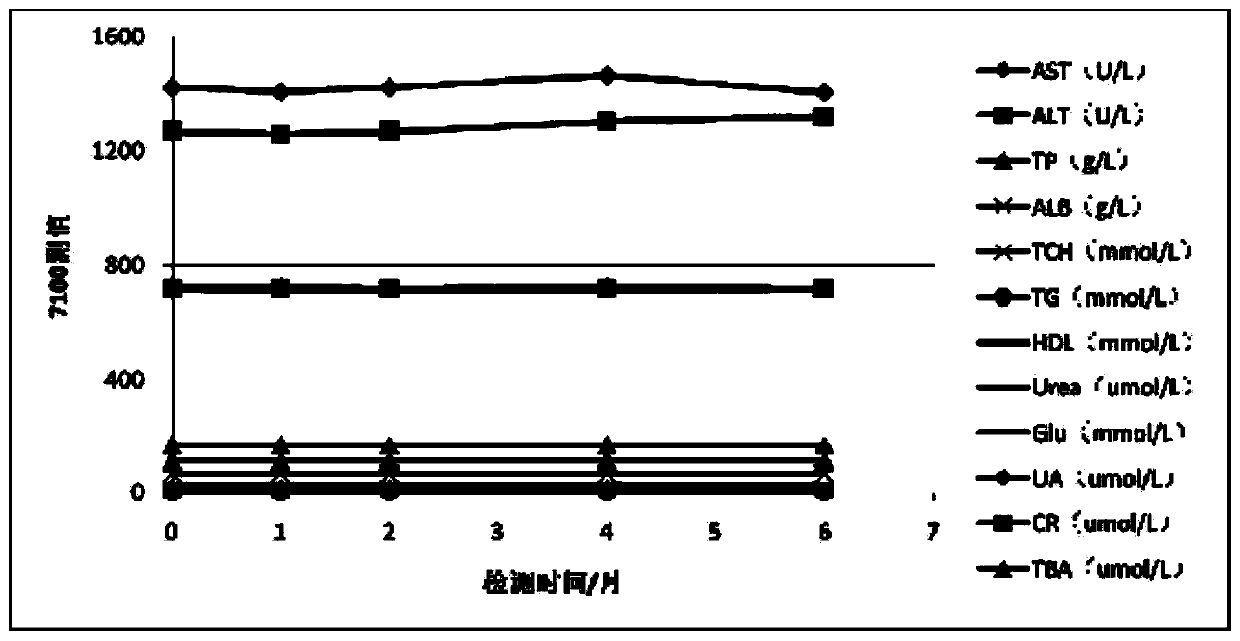
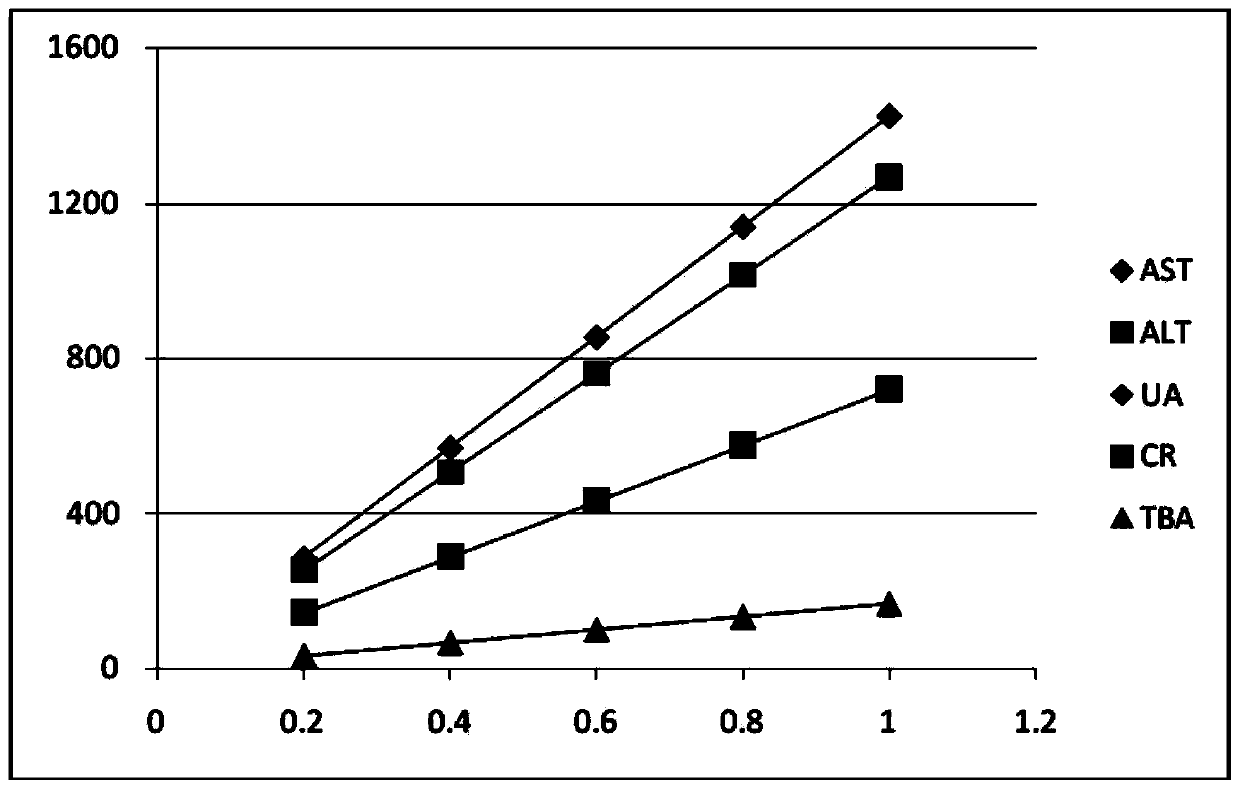
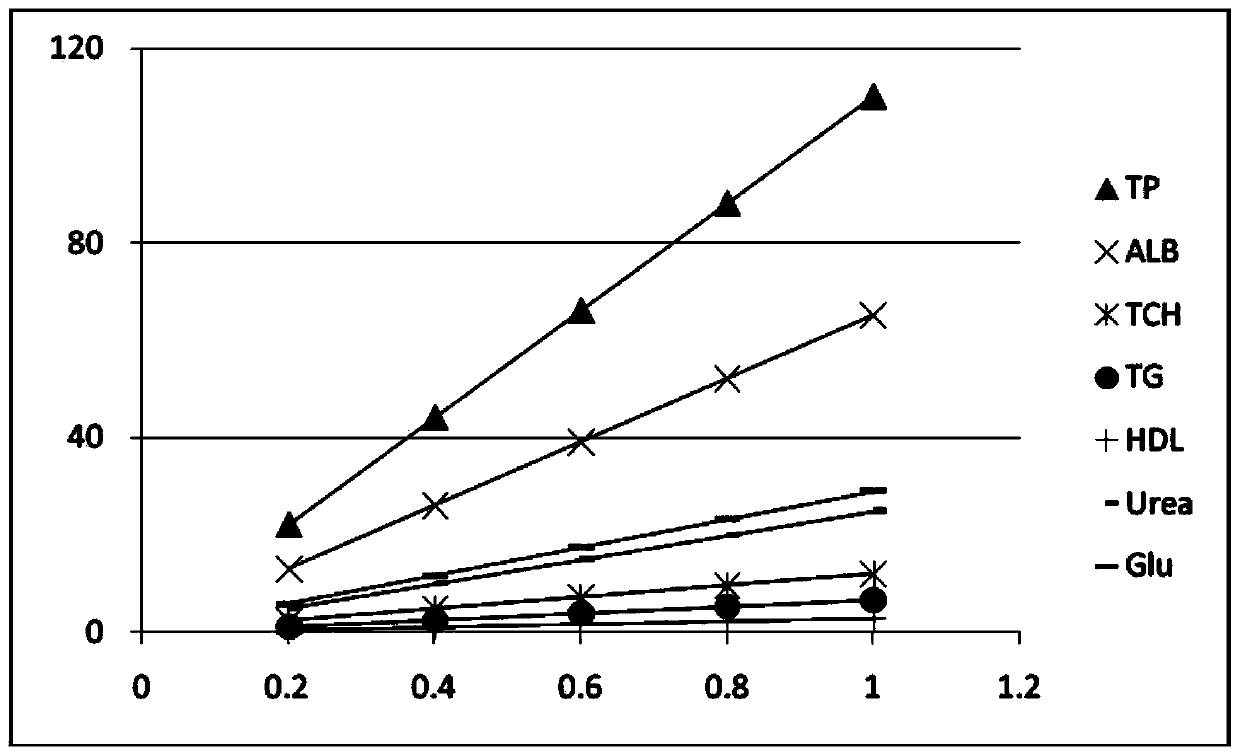
[0033] The mixed linear high values (sample) of the above examples were measur...
Embodiment 2
[0045] 1. Collect the serum of the health examination, the hepatitis B surface antibody, hepatitis A antibody, hepatitis C antibody, HIV antibody, etc. are all negative, and the serum is divided into two (equal volume);
[0046] 2. Add 0.9% cholesteryl sulfate sodium salt to one of the serums, heat to 60°C, and cool to room temperature after completely dissolving;
[0047] 3. Add 0.3% urea, 0.03% creatinine, 0.04% uric acid, 0.9% glucose, 0.04% glycocholic acid, and 0.09% triolein to another serum in sequence;
[0048] 4. Mix the solutions obtained in step 2 and step 3;
[0049] 5. Add 5% bovine serum albumin, 700U / L alanine aminotransferase, and 750U / L aspartate aminotransferase to the solution obtained in step 4;
[0050] 6. Add 6% Tween 80 and 0.15% PC300 to the solution obtained in step 5; after fully dissolving and mixing, subpackage and freeze-dry.
[0051] The stability of the mixed linear high values of the above examples was investigated. The test was carried out...
Embodiment 3
[0053]1. Collect the serum of the health examination, the hepatitis B surface antibody, hepatitis A antibody, hepatitis C antibody, HIV antibody, etc. are all negative, and the serum is divided into two (equal volume);
[0054] 2. Add 1.0% cholesteryl sulfate sodium salt to one of the serums, heat to 65°C, and cool to room temperature after completely dissolving;
[0055] 3. Add 0.5% urea, 0.05% creatinine, 0.05% uric acid, 1.2% glucose, 0.05% glycocholic acid, and 0.12% triolein to another serum in sequence;
[0056] 4. Mix the solutions obtained in step 2 and step 3;
[0057] 5. Add 6% bovine serum albumin, 800U / L alanine aminotransferase, and 800U / L aspartate aminotransferase to the solution obtained in step 4;
[0058] 6. Add 5% sucrose, 0.5% Tween 80, and 0.2% sodium azide to the solution obtained in step 5; after fully dissolving and mixing, subpackage and freeze-dry.
[0059] The mixed linear high values of the above examples are used to evaluate the linear range of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com