A kind of synthetic method of lenvatinib
A technology of lenvatinib and its synthetic method, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of difficult purchase, limited application, complicated synthesis, etc., and achieve the effects of simple operation, low requirements for production equipment and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
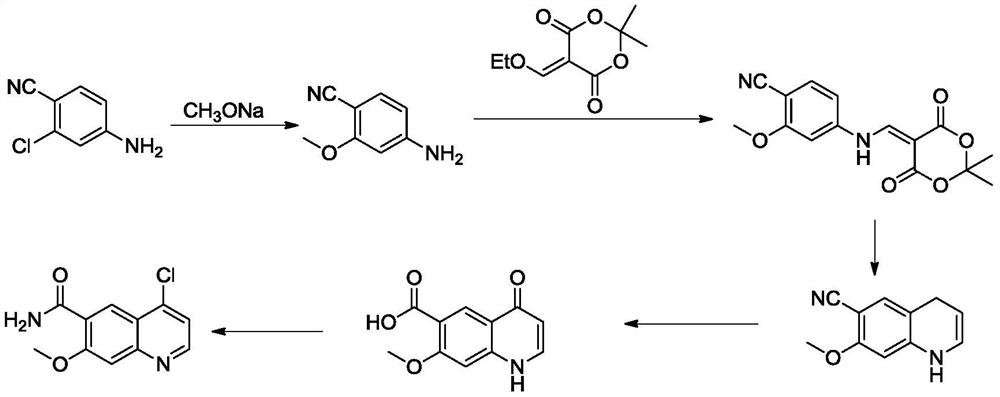
[0044] A kind of synthetic method of lenvatinib, concrete steps are as follows:
[0045] Step 1, the synthesis of 4-cyano-3-methoxyaniline: Take 134g of 4-cyano-3-hydroxyaniline, dissolve it in 500mL N, N-dimethylformamide (DMF) and mix well, then take 300g of the mixture , add 32g of tetrabutylammonium bromide, 268g of potassium carbonate, the temperature of the reaction system is raised to 110°C, and 100g of dimethyl carbonate is added dropwise. After the dropwise addition, the reaction is continued for 8h. Remove the solvent by distillation under pressure, add 200g of water, extract with 500mL of ethyl acetate, dry the organic layer over anhydrous sodium sulfate, filter, recover the solvent from the filtrate, and recrystallize the residue with a mixture of petroleum ether and ethyl acetate. The volume ratio was 1:1300, and 139 g of light yellow solid was obtained.
[0046] Step 2, the synthesis of oxime: Take 74g of the first step product 4-cyano-3-methoxyaniline, dissolve...
Embodiment 2
[0054] Step 1, the synthesis of 4-cyano-3-methoxyaniline: Take 100g of 4-cyano-3-hydroxyaniline, dissolve it in 500mL DMF and mix evenly, take 300g of the mixture, add 25g of tetrabutylammonium bromide, carbonic acid Potassium 200g, the temperature of the reaction system was raised to 110°C, and 100g of dimethyl carbonate was added dropwise. After the dropwise addition, the reaction was continued for 7h. After the reaction, the operation was the same as in Example 1 to obtain 103g of a light yellow solid.
[0055] Step 2, the synthesis of oxime: take 50g of 4-cyano-3-methoxyaniline, dissolve in ethanol, heat to reflux, add 50g of propionic acid dropwise, after the dropwise addition, continue to react for 4h, after the end of the reaction, reduce Recover the solvent by pressure. The residue was continued to the next step without separation.
[0056] Step 3, the synthesis of 6-cyano-7-methoxy-4-quinolinone: add 100mL polyphosphoric acid to the residue of the previous step react...
Embodiment 3
[0063] Step 1, the synthesis of 4-cyano-3-methoxyaniline: Take 150g of 4-cyano-3-hydroxyaniline, dissolve it in 500mL DMF and mix evenly, take 300g of the mixture, add 34g of tetrabutylammonium bromide, carbonic acid Potassium 300g, the temperature of the reaction system was raised to 110°C, and 100g of dimethyl carbonate was added dropwise. After the dropwise addition, the reaction was continued for 9h. After the reaction, the operation was the same as in Example 1 to obtain 146g of a light yellow solid.
[0064] Step 2, the synthesis of oxime: take 100g of 4-cyano-3-methoxyaniline, dissolve in ethanol, heat to reflux, add 50g of propionic acid dropwise, after the dropwise addition, continue the reaction for 6h, after the reaction, reduce Recover the solvent by pressure. The residue was continued to the next step without separation.
[0065] Step 3, the synthesis of 6-cyano-7-methoxy-4-quinolinone: add 100mL polyphosphoric acid to the residue of the previous step reaction, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com