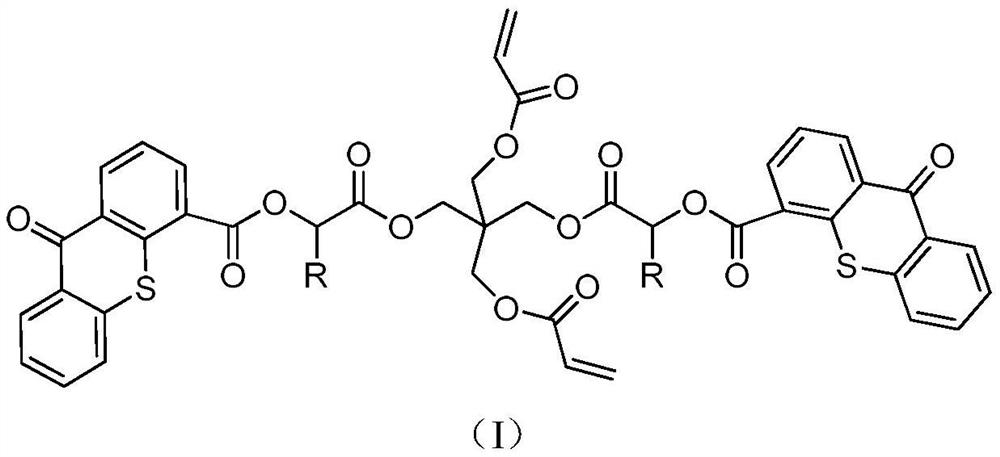
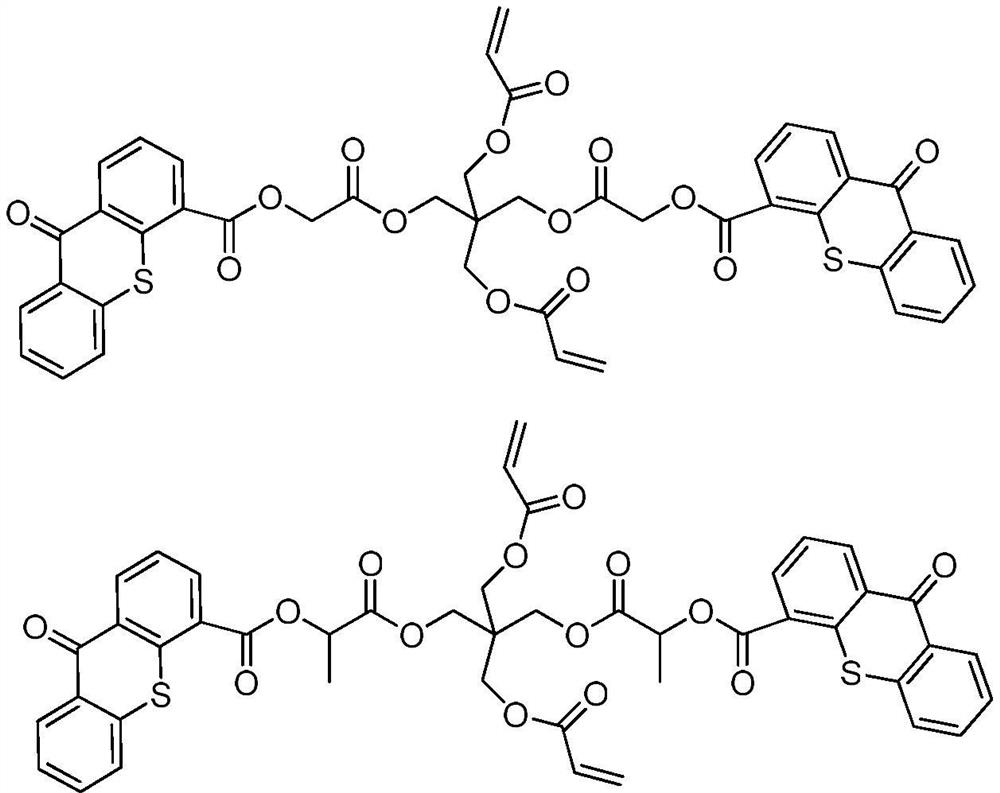
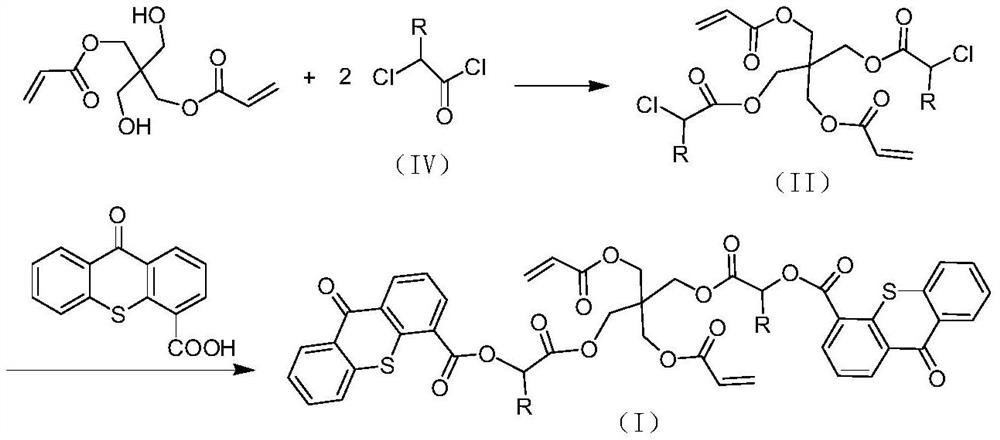
A kind of polymerizable type II photoinitiator and preparation method thereof
A technology for polymerizing photoinitiators and inhibitors, which is applied in the field of polymerizable type II photoinitiators and its preparation, can solve problems such as toxicity and deterioration of physical properties of packaging materials, and achieve low mobility, excellent photocuring performance, and relative good capacitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]Example 1:Preparation of compound A
[0032]Add 48.8g of pentaerythritol diacrylate (0.2mol), 23.3g of triethylamine (0.21mol) and 240ml of dichloromethane into the three-necked flask, electromagnetic stirring reaction, the three-necked flask is connected to the thermometer, drying tube and A constant pressure dropping funnel containing 46.3g of chloroacetyl chloride (0.41mol) and 120ml of dichloromethane was slowly added dropwise at low temperature, and the temperature was kept between 0 to -5°C. After the addition, it was moved to room temperature for reaction. TLC monitors the completion of the reaction. Filter triethylamine hydrochloride, wash with saturated sodium bicarbonate 2-3 times, then with saturated brine, dry with anhydrous magnesium sulfate, and spin dry. Separation and purification by a chromatographic column gives a transparent oily substance with a yield of about 85% and a purity of ≥95%.1H NMR(400MHz, CDCl3) δ 6.45 (dd, 1H), 6.05 (m, 2H), 5.58 (dd, 2H), 4.34 (s,...
Embodiment 2
[0034]Example 2:Preparation of compound B
[0035]Add 48.8g of pentaerythritol di(acrylic acid) ester (0.2mol), 23.3g of triethylamine (0.21mol) and 240ml of dichloromethane into the three-necked flask, electromagnetic stirring reaction, the three ports of the three-necked flask are respectively connected to a thermometer and a drying tube And a constant pressure dropping funnel containing 51.6g 2-chloropropionyl chloride (0.41mol) and 120ml dichloromethane, slowly drip at low temperature, keep the temperature between 0 and -5℃, after the addition, move to Reaction at room temperature. TLC monitors the completion of the reaction. Filter triethylamine hydrochloride, wash with saturated sodium bicarbonate 2-3 times, then with saturated brine, dry with anhydrous magnesium sulfate, and spin dry. Separation and purification by a chromatographic column gives a transparent oily substance with a yield of about 80% and a purity of ≥95%.1H NMR(400MHz, CDCl3)δ6.37(dd,1H),6.15(m,2H),5.54(dd,2H),4....
Embodiment 3
[0037]Example 3:Preparation
[0038]In a 500ml four-neck flask equipped with mechanical stirring, add 50.0g thioxanthone-4-carboxylic acid, 250ml tetrahydrofuran, 23.6g triethylamine, 25.6g methyl chloroacetate, and heat to 50~60℃ to react for 8 hours. After suction filtration, the filtrate was cooled to 0-5°C and stirred and crystallized for 2 hours to obtain a crude product. The crude product was recrystallized with toluene. After drying, 53.7g of yellow flake crystals were obtained. The yield was 84% and the content was ≥98.0%.
[0039]In a 500ml four-necked flask equipped with mechanical stirring and water separator, add 32.8g thioxanthone-4-formyloxyacetic acid methyl ester, 0.3g sodium methoxide, 200ml methylcyclohexane, 13.5g pentaerythritol diacrylic acid Ester, 0.2g p-hydroxyanisole, heated to reflux and separated methanol for 14 hours. Slowly lower the temperature, precipitate solids, and filter to obtain 24.2 g of light yellow solids with liquid content ≥92%. MS: m / z[M+1]+= 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com