Catalpol derivative and application thereof
A derivative, catalpol technology, applied in the field of medicine, can solve unrealistic problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
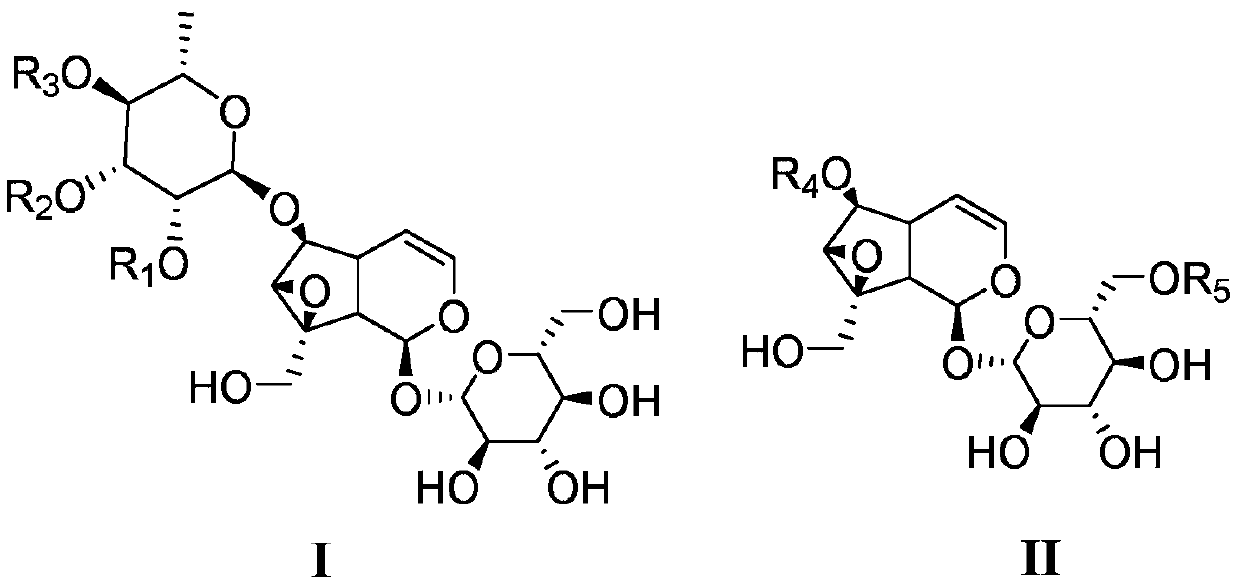
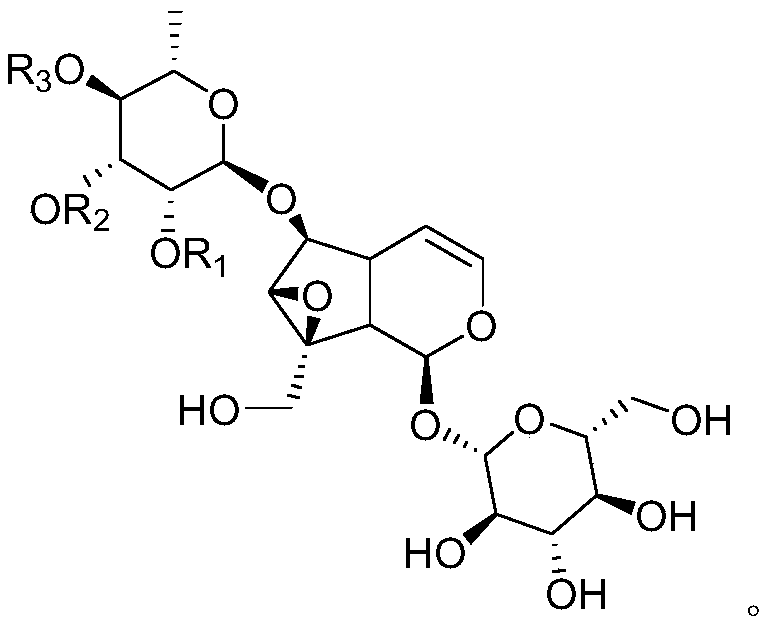
[0059] Embodiment 1: the preparation of formula I natural catalpol derivative
[0060] Extract 4.1 kg of Scrophularia serrata medicinal material with 95% ethanol aqueous solution by reflux for 3 times, each time for 2 hours, each time with 40 L of ethanol aqueous solution, combine the extracts, concentrate under reduced pressure to obtain extract (about 570 g); Add about 3 times the amount (1500mL) of water to the extract to suspend, then extract with petroleum ether (1500mL×3) and ethyl acetate (1500mL×3) in sequence, collect the ethyl acetate extract; recover ethyl acetate under reduced pressure Carry out silica gel column chromatography after esterification, carry out gradient elution with petroleum ether and ethyl acetate successively (10:1~0:1); Use silica gel column chromatography of the obtained fraction: first carry out gradient with petroleum ether-ethanol mixed solvent Elution (volume ratio from 10:1 to 3:1), then reverse column chromatography: gradient elution with ...
Embodiment 2
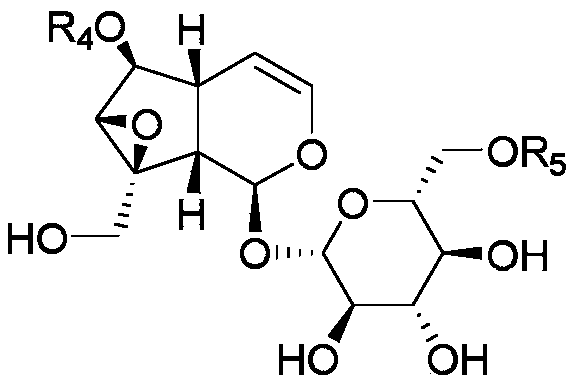
[0086] Embodiment 2: the preparation of formula II natural catalpol derivative
[0087] Rhizome of Coptis chinensis 1kg, crushed into coarse powder, heated and refluxed with 10 times the amount of methanol to extract 3 times, concentrated and then added 200-300 mesh silica gel to mix the sample, 300-400 mesh silica gel column chromatography, chloroform-methanol (1:20 -1:5) gradient elution to obtain 2 fractions. Wherein Fraction 2 is chromatographed on a reversed-phase ODS column, eluted with a gradient of methanol-water (10%-30%), and the fraction of the resulting Pibroside II fraction is purified by a sephadex LH-20 column (50% methanol), and the II -1~II-6, all are white powder.
[0088] 10 kg of catalpa, crushed into coarse powder, heated and refluxed with 10 times the amount of 75% ethanol for 3 times, each time for 2 hours, the extract was decompressed to recover the ethanol until it had no alcohol smell, and extracted 3 times with the same amount of dichloromethane to ...
Embodiment 3
[0120] Embodiment 3 Catalbin is structurally modified to obtain the preparation of catalpol derivatives
[0121] Add catalpaside 24mg (0.05mmol) and 3mL N,N-dimethylformamide (DMF) into the reaction flask, add R under stirring at room temperature 1 X (0.10mmol) (Table 1) and anhydrous K 2 CO 3 (21mg, 0.15mmol), reacted at 30°C to the end point (TLC tracking). An appropriate amount of 100-200 mesh silica gel was added to mix the sample, and the solvent was recovered under reduced pressure until it was in the shape of quicksand, and the corresponding compound of formula III was obtained by silica gel and MCI column chromatography, with a yield between 50% and 98%.
[0122] Table 1 prepares the substrate reagent of necatapol derivative
[0123]
[0124]
[0125] III-1: 1 H NMR (600 MHz, C 5 D. 5 N): δ H 5.53(1H,d,J=9.1 Hz,H-1),6.43(1H,dd,J=1.4 / 5.9 Hz,H-3),5.06(1H,dd,J=5.9 / 4.1 Hz,H-4 ),2.91(1H,m,H-5),5.28(1H,brd,J=7.6 Hz,H-6),3.97(1H,brs,H-7),2.88(1H,m,H-9) ,4.50(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com