Method for quantitatively determining flavonoid compounds
A technology for quantitative determination of flavonoids, applied in material excitation analysis, fluorescence/phosphorescence, etc., can solve the problems of inconvenient on-site detection, high cost, and complexity, and achieve high sensitivity, fast response speed, and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
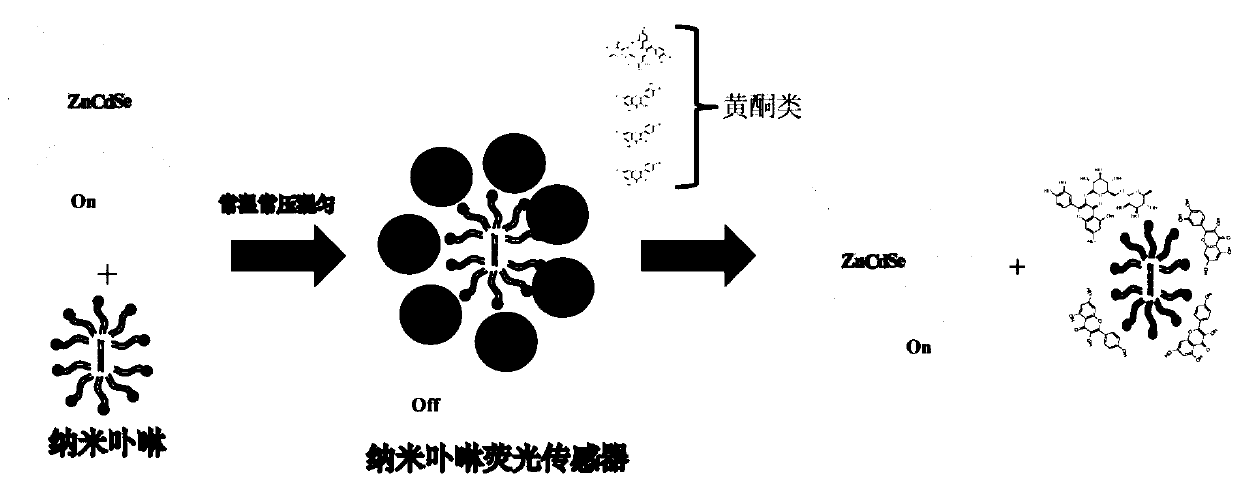
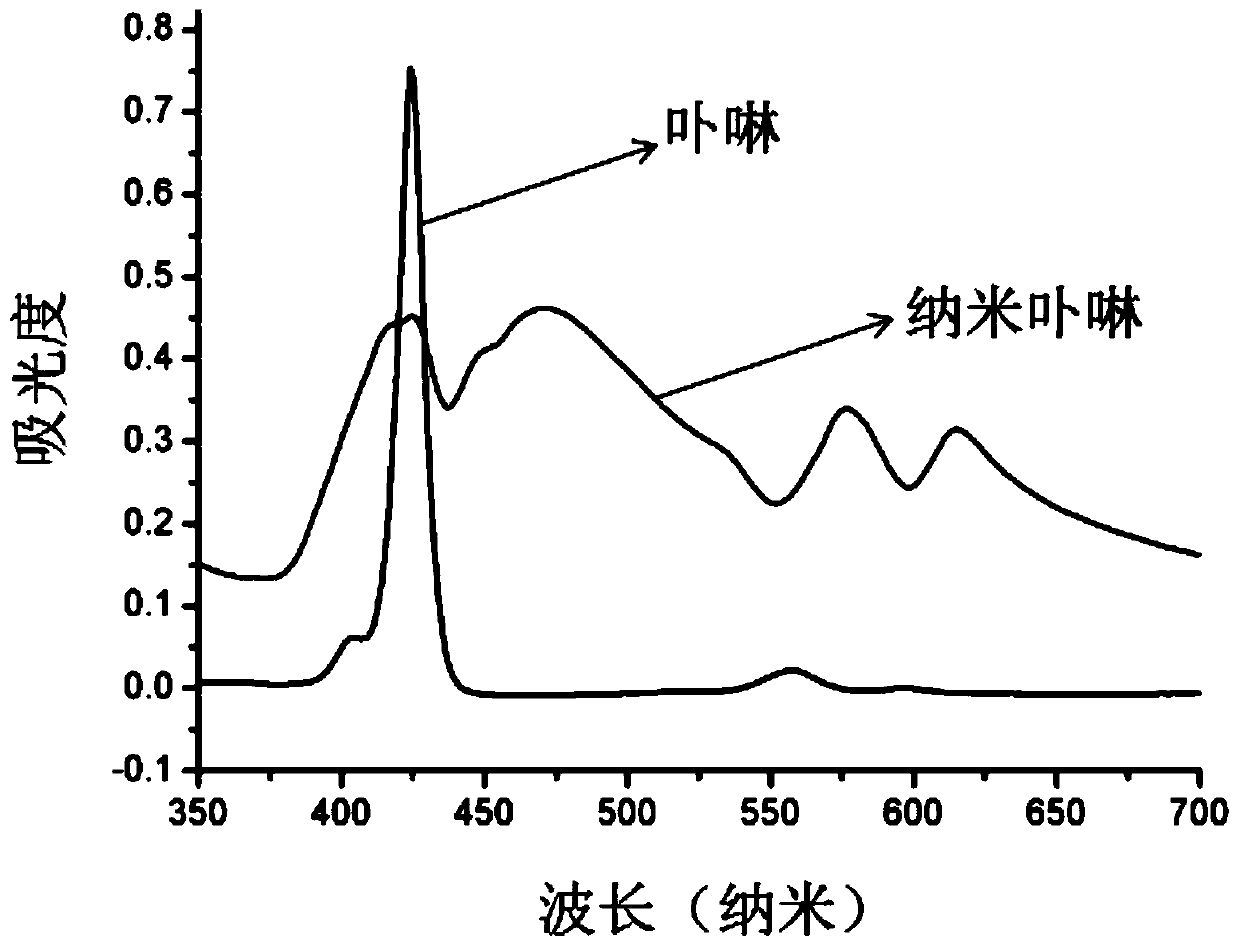
[0043] Embodiment 1: The recognition and quantitative analysis of quercetin by the reversible nano-porphyrin fluorescent sensor, the schematic diagram of the method is shown in 1, and the steps are as follows:
[0044] (1) Synthesis of ZnCdSe quantum dot fluorescent probe
[0045] Dissolve zinc dichloride (0.035g, 6.4mM) and N-acetyl-L-cysteine (0.1253g, 19.2mM) in 40mL of ultrapure water, stir for 20 minutes in an ice bath, and use Adjust the pH of the solution to 9.7 with sodium hydroxide solution, adjust the pH by adding 100 μL of cadmium dichloride (0.00058 g, 0.237 mM), then fill with nitrogen and stir in an ice bath for 5 to 10 minutes. NaHSe was added and stirred for 5 minutes. Finally, the solution was put into a reaction kettle and reacted in an oven at 200° C. for 65 minutes. Cooling to room temperature yielded 4.9×10 -9 mol / LZnCdSe quantum dot fluorescent probe.
[0046] (2) Synthesis of nanoporphyrin solution
[0047] Dissolve an appropriate amount of tetrak...
Embodiment 2
[0054] Embodiment 2: Quantitative analysis of rutin by a reversible nanoporphyrin fluorescent sensor, the schematic diagram of the method is shown in 1, and the steps are as follows:
[0055] (1) Synthesis of ZnCdSe quantum dot fluorescent probe
[0056] The ZnCdSe quantum dot fluorescent probe was synthesized by the method of step (1) in Example 1.
[0057] (2) Synthesis of tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution
[0058] The method of step (2) in Example 1 was used to synthesize tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution.
[0059] (3) Preparation of switch nanoporphyrin fluorescent sensor
[0060] The nano-porphyrin fluorescence sensor was prepared by the method of step (3) in Example 1.
[0061] (4) Quantitative analysis of rutin with reversible nanoporphyrin fluorescent sensor
[0062] Add 100 μL rutin aqueous solution to 1.5mL cuvette, 300 μL 1.68×10 -5mol / L tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution synthesized in step...
Embodiment 3
[0063] Embodiment 3: Quantitative analysis of kaempferol by reversible nanoporphyrin fluorescent sensor, the schematic diagram of the method is shown in 1, and the steps are as follows:
[0064] (1) Synthesis of ZnCdSe quantum dot fluorescent probe
[0065] The ZnCdSe quantum dot fluorescent probe was synthesized by the method of step (1) in Example 1.
[0066] (2) Synthesis of tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution
[0067] The method of step (2) in Example 1 was used to synthesize tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution.
[0068] (3) Preparation of switch nanoporphyrin fluorescent sensor
[0069] The nano-porphyrin fluorescence sensor was prepared by the method of step (3) in Example 1.
[0070] (4) Quantitative analysis of kaempferol by reversible nanoporphyrin fluorescent sensor
[0071] Add 100 μL kaempferol aqueous solution to 1.5mL cuvette, 300 μL 1.68×10 -5 mol / L tetrakis-(4-pyridyl)zinc porphyrin self-assembly solution synthes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com