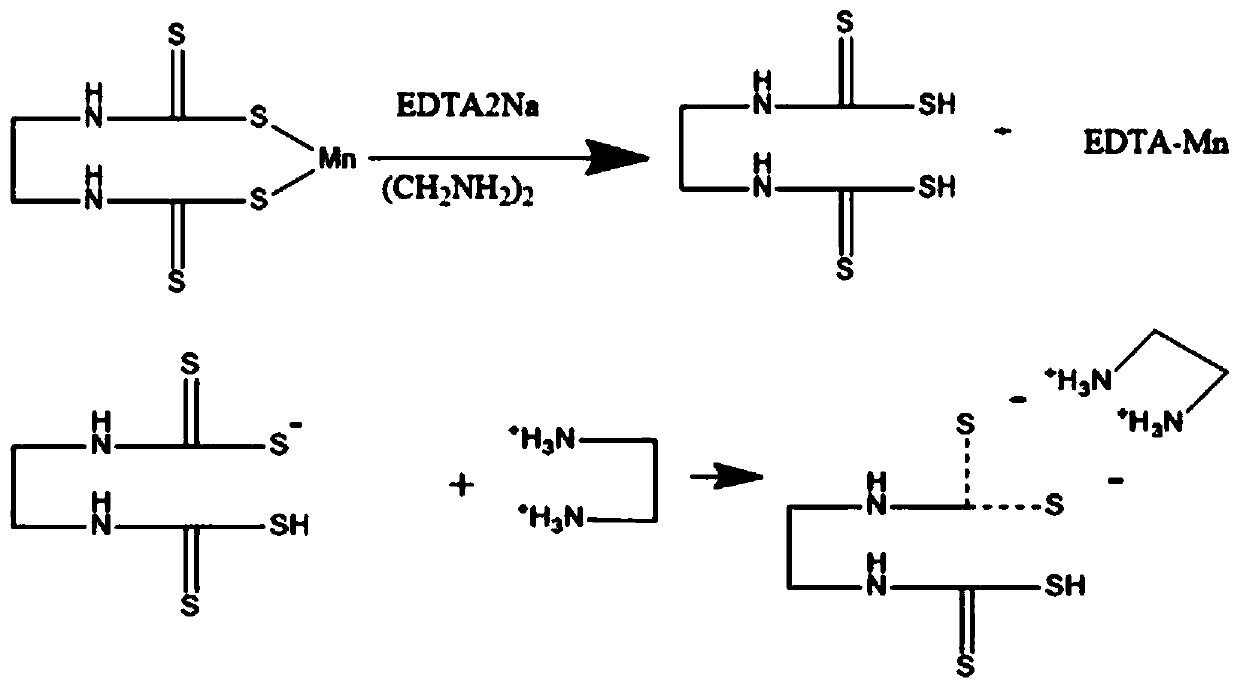
Non-derivative detection method for ethylene bisdithiocarbamates
The technology of a dithioamino group and a detection method is applied in the non-derivatized detection field of ethylene bis-dithiocarbamate pesticides, which can solve the problems of complicated and complicated operations, and achieve simple pretreatment, high reproducibility, and sensitivity. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 A kind of detection method of dyson pesticide
[0062] Preparation of standard solution: Weigh 0.001g of Mancozeb, Maneb, Zinc, Mancozeb, Sodium, Propineb, and Metabam respectively in a 10ml volumetric flask, and add 0.01g of ethylenediamine As a solid disodium tetraacetate, dissolve it with 3% aqueous solution of ethylenediamine, and dilute to the mark. Accurately draw appropriate amounts of 9 standard stock solutions, put them in a 10ml volumetric flask, and gradually dilute them with 3% ethylenediamine aqueous solution to form a mixed standard working solution of appropriate concentration.
[0063] Preparation of solution: Weigh 0.1g of EDTA2Na solid into a 100mL volumetric flask, add 5mL of ethylenediamine solution, and dilute to 100mL with ultrapure water.
[0064] Weigh 5.0g of the sample to be tested in a 50ml centrifuge tube, soak twice in 20ml of water, vortex for 10min, let stand for 10min, and filter with a filter; soak twice in 20ml of carbon d...
Embodiment 2
[0074] The selection of embodiment 2 solvent
[0075] 1. Experimental method
[0076] The dyson pesticides were dissolved with 3% ethylenediamine methanol solution of EDTA and 3% ethylenediamine aqueous solution of EDTA respectively, and the stability of dyson pesticides were investigated at 1h, 2h, 1Day and 1Week.
[0077] 2. Experimental results
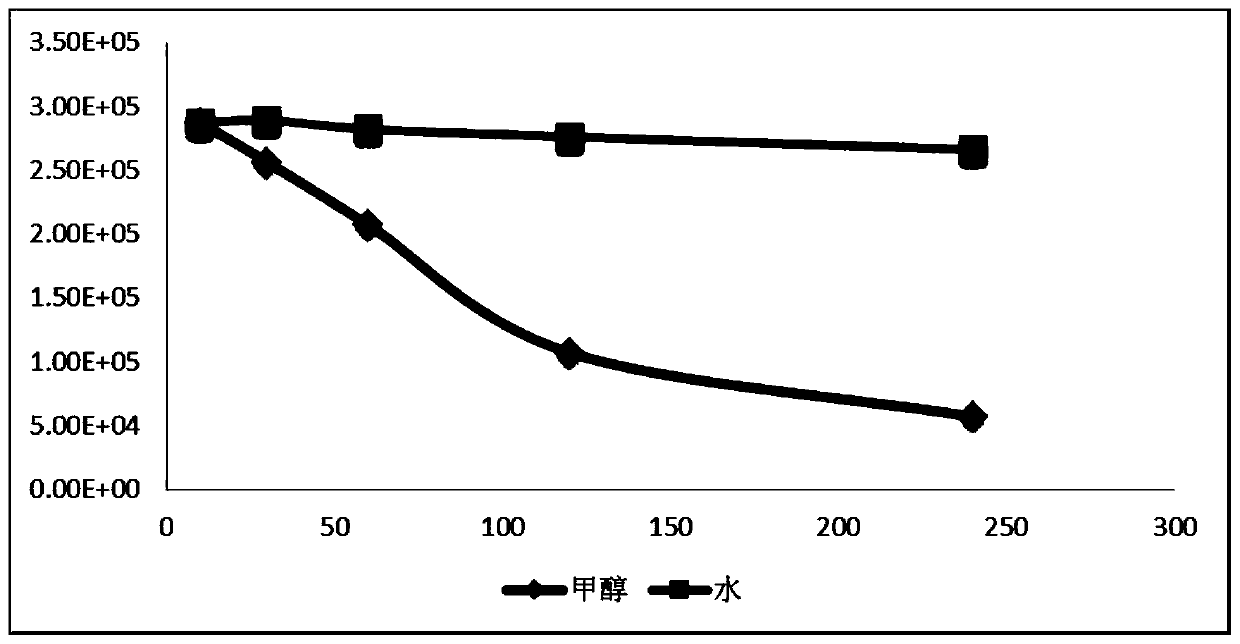
[0078] The results are shown in Table 2 and figure 2 , the results showed that 3% ethylenediamine methanol solution of EDTA could dissolve the dyson, but the dyson quickly decayed to nothing within 2 hours. However, the dysons in 3% ethylenediamine aqueous solution of EDTA can remain stable for 60 days. Therefore water was chosen as the solvent.
[0079] The influence of different solvents of table 2 on the dissolution of Dyson:
[0080]
Embodiment 3
[0081] The influence of different amines of embodiment 3 on the solubility of dysons in EDTA aqueous solution
[0082] 1. Experimental method
[0083] Use ethylenediamine, isobutylamine, dimethylamine, ammonia water, di-n-butylamine, and pyridine to dissolve dyson pesticides in EDTA aqueous solution respectively, and detect them on the machine. Solubility in EDTA aqueous solution.
[0084] 2. Experimental results
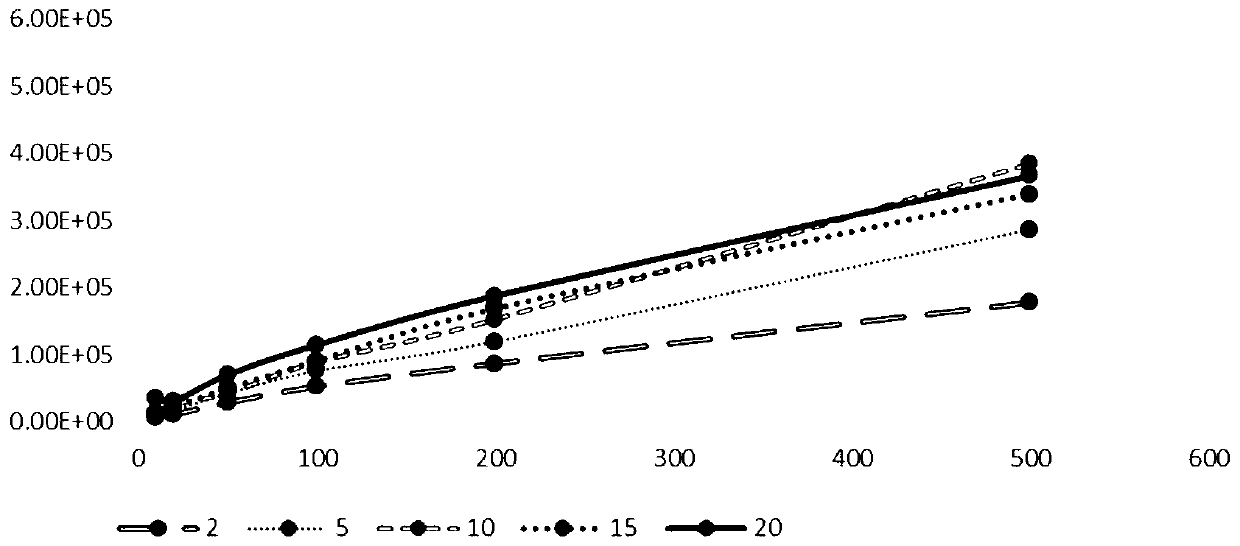
[0085] The results are shown in Table 3. Ethylenediamine can ionize dysonins and can be broken into fragment ions in mass spectrometry, while other amines cannot ionize dysonins. Therefore, ethylenediamine was selected as the dissolving agent of Daisen.
[0086] The influence of different amines of table 3 on the dissolution of Dyson:
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com