Preparation method of a network-like carbon-supported iron-based compound material and its application in lithium-sulfur batteries
A lithium-sulfur battery and network-like technology, applied in the field of electrochemistry, can solve problems such as the lack of in-depth research on catalysis, the suppression of the shuttle effect of difficult polysulfide compounds, and the reduction of the use of noble metal catalysts, so as to increase the effect of sulfur fixation and suppress the shuttle effect , The synthetic method is simple and convenient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
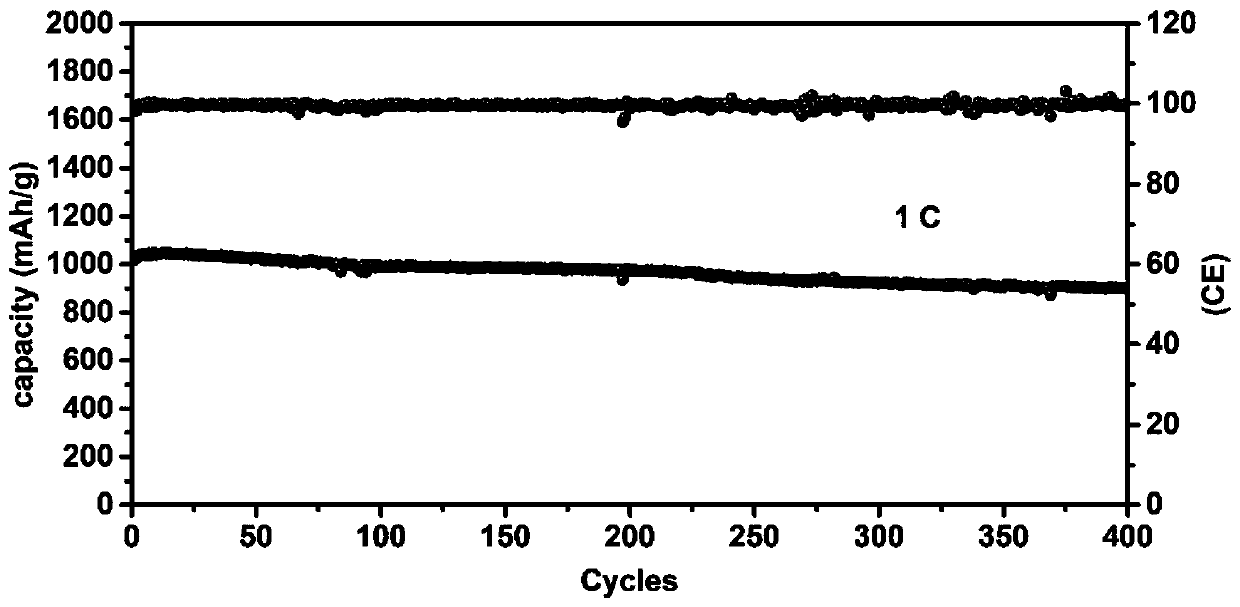
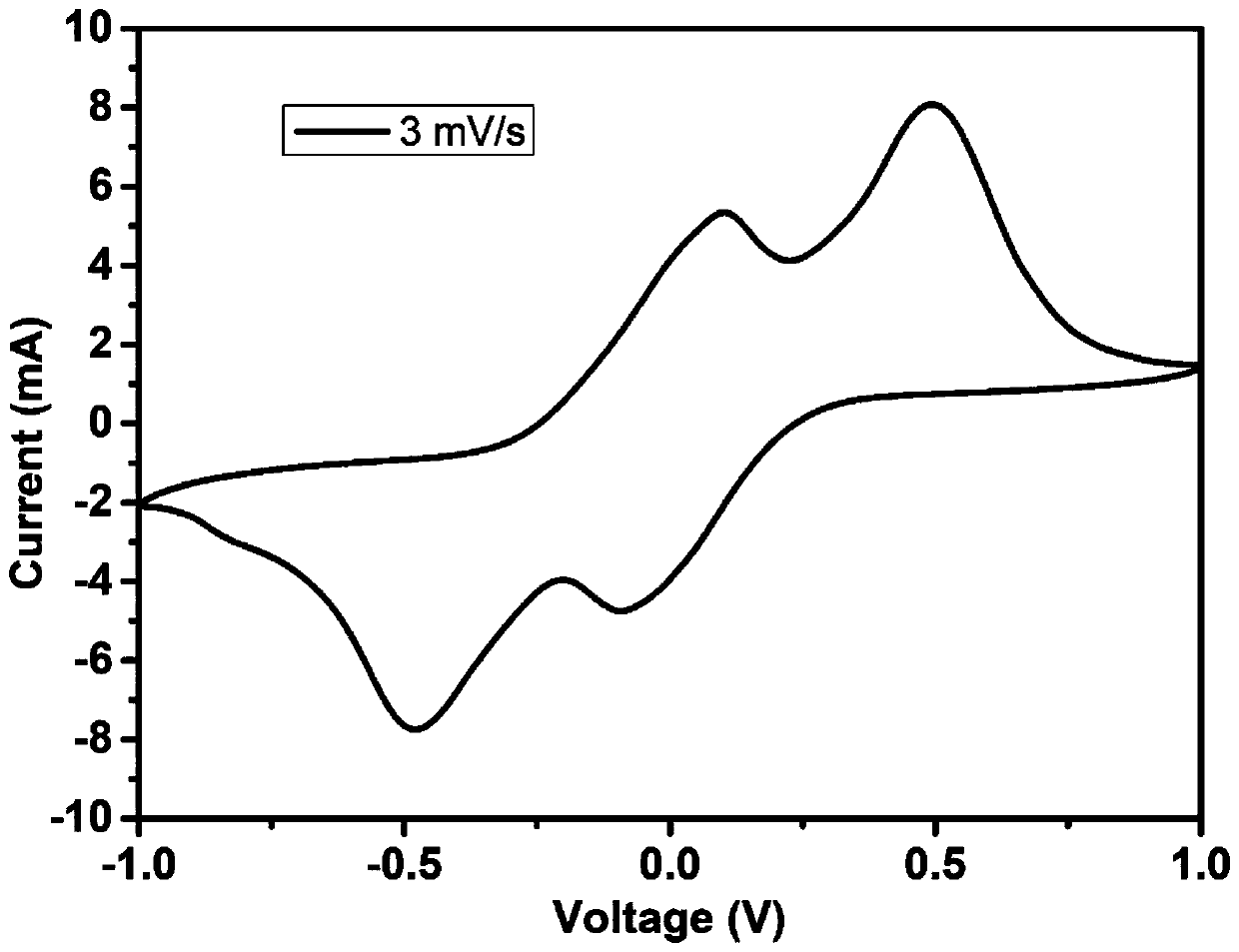
[0030] At room temperature, 1.0050 g of ferric nitrate nonahydrate and 10 mL of GO (5 mg / mL) were added to 40 mL of deionized water, sonicated for 2 hours and stirred for 12 hours. 1.0052 g of glucose was added and stirring was continued for 8 hours at room temperature. The resulting homogeneous mixture was transferred into a hydrothermal kettle and kept at 180°C for 10 hours. After the reaction kettle was lowered to room temperature, it was suction-filtered, washed three times with deionized water, and dried at 60°C. The dried product was thoroughly ground and mixed with 10 times the mass of melamine, and then kept at 900°C for 1 hour under the protection of argon with a heating rate of 5°C / min. When the temperature drops to room temperature, mix the resulting material and binder (PVDF) at a ratio of 9:1, add NMP and stir at room temperature for 12 hours to form a uniform slurry, which is coated on a commercial PP separator with a thickness of 10 microns and heated at 80°C D...
Embodiment example 2
[0036] At room temperature, 1.5 g of ferric nitrate nonahydrate and 20 mL of GO (5 mg / mL) were added to 40 mL of deionized water, sonicated for 1 hour and stirred for 8 hours. 2 g of glucose were added and stirring was continued for 12 hours at room temperature. The resulting homogeneous mixture was transferred into a hydrothermal kettle and kept at 160°C for 10 hours. After the reaction kettle was lowered to room temperature, it was suction-filtered, washed three times with deionized water, and dried at 60°C. The dried product was thoroughly ground and mixed with 8 times the mass of melamine, and then kept at 800°C for 4 hours under the protection of argon with a heating rate of 5°C / min. When the temperature drops to room temperature, mix the resulting material and binder (PVDF) at a ratio of 9:1, add NMP and stir at room temperature for 12 hours to form a uniform slurry, which is coated on a commercial PP separator with a thickness of 10 microns and heated at 80°C Dry for 1...
Embodiment example 3
[0042] At room temperature, 0.5 g of ferric nitrate nonahydrate and 15 mL of GO (5 mg / mL) were added to 40 mL of deionized water, sonicated for 3 hours and stirred for 10 hours. 0.5 g of glucose was added and stirring was continued for 10 hours at room temperature. The resulting homogeneous mixture was transferred into a hydrothermal kettle and kept at 170°C for 13 hours. After the reaction kettle was lowered to room temperature, it was suction-filtered, washed three times with deionized water, and dried at 60°C. The dried product was thoroughly ground and mixed with 6 times the mass of melamine, and then kept at 1000°C for 2 hours under the protection of argon with a heating rate of 5°C / min. When the temperature drops to room temperature, mix the resulting material and binder (PVDF) at a ratio of 9:1, add NMP and stir at room temperature for 12 hours to form a uniform slurry, which is coated on a commercial PP separator with a thickness of 10 microns and heated at 80°C Dry f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com