Universal method for preparing porous carbon material based on organic zinc salt
A porous carbon material, organic zinc technology, applied in carbon preparation/purification, etc., can solve problems such as equipment corrosion, achieve high capacitance value, easy to scale up production, and enrich the effect of ultra-microporous structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
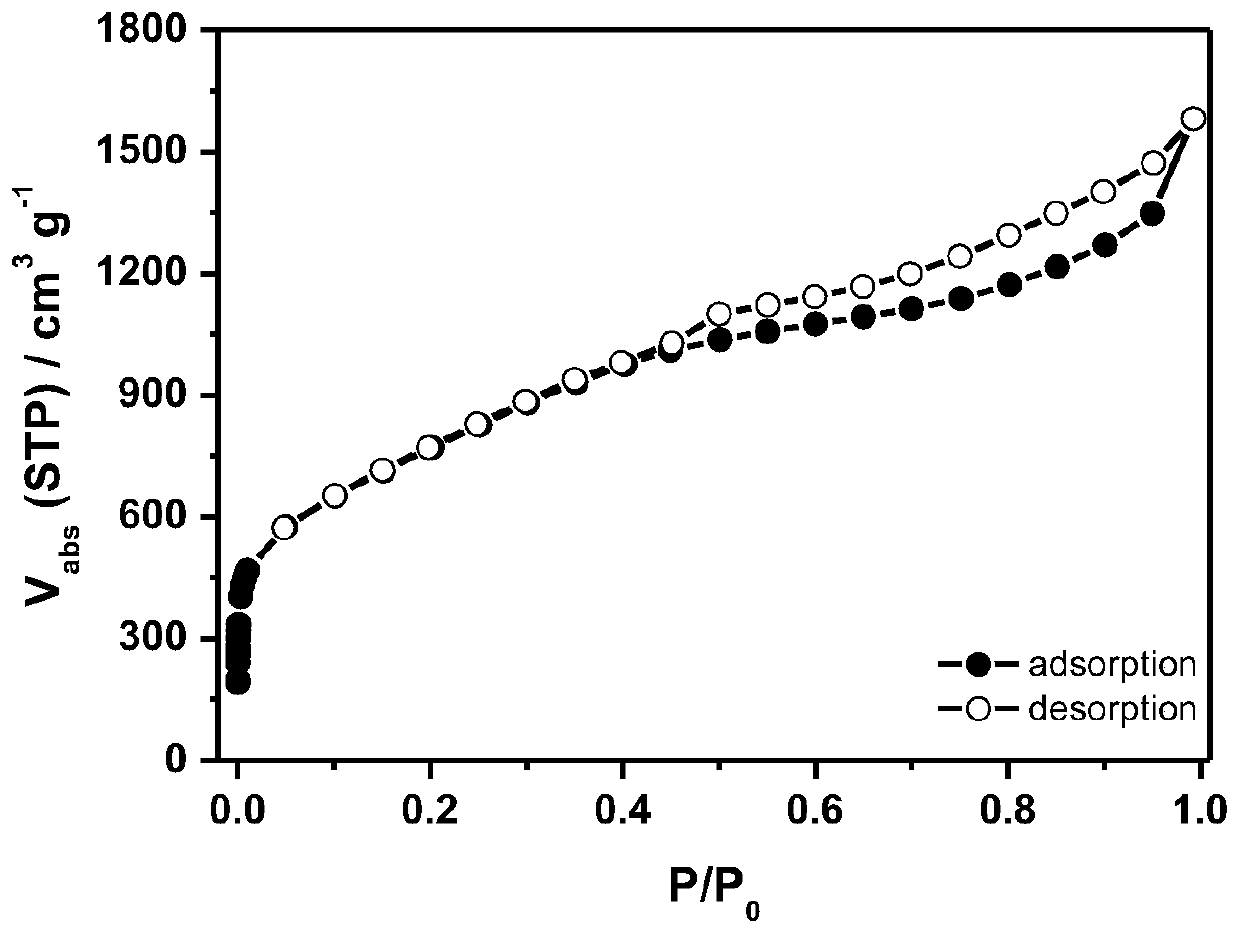
Image
Examples
Embodiment 1
[0021] Prepare porous carbon materials according to the following steps:
[0022] Step one: with zinc citrate (C 12 h 10 o 14 Zn 3 ) as carbon precursors.
[0023] Step 2: Weigh 1.00 grams of zinc citrate and place it evenly in 1*3cm 2 The bottom of the quartz boat ensures that the sample is heated evenly and can complete the carbonization and Zn volatilization process in a short period of time, and the temperature is slowly programmed from room temperature to 1000 °C;
[0024] Step 3: Control the temperature of the zinc salt material at 1000°C while maintaining the nitrogen flow, and the activation time is 2 hours.
[0025] Step 4: Cool to room temperature under nitrogen flow to obtain porous carbon material directly.
[0026] The temperature programming rate described in step 2 is 5°C / min. The nitrogen flow rate described in step 3 is 50 ml per minute.
[0027] The present invention has no strict restrictions on the heating equipment of the carbonization process, suc...
Embodiment 2
[0030] Step 1: Zinc gluconate is used as carbon precursor.
[0031]Step 2: Weigh 1.00 grams of zinc gluconate and place it evenly in 1*3cm 2 The bottom of the quartz boat ensures that the sample is heated evenly and can complete the carbonization and Zn volatilization process in a short period of time, and the temperature is slowly programmed from room temperature to 1000 °C;
[0032] Step 3: Under the nitrogen flow, the temperature of the zinc salt material is controlled at 1000° C., and the activation time is 3 hours.
[0033] Step 4: Cool to room temperature under nitrogen flow to obtain porous carbon material directly.
[0034] The temperature programming rate described in step 2 is 2°C / min. The nitrogen flow rate described in step 3 is 100 ml per minute.
Embodiment 3
[0036] Step 1: Zinc glycinate is used as carbon precursor.
[0037] Step 2: Weigh 1.00 grams of zinc glycinate and place it evenly in 1*3cm 2 The bottom of the quartz boat ensures that the sample is heated evenly and can complete the carbonization and Zn volatilization process in a short period of time, and the temperature is slowly programmed from room temperature to 1000 °C;
[0038] Step 3: Control the temperature of the zinc salt material at 1000°C while maintaining the nitrogen flow, and the activation time is 2 hours.
[0039] Step 4: Cool to room temperature under nitrogen flow to obtain porous carbon material directly.
[0040] The temperature programming rate described in step 2 is 10°C / min. The nitrogen flow rate described in step 3 is 80 ml per minute.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com