Method for preparing lithium hydroxide from waste lithium-ion batteries
A lithium-ion battery, lithium hydroxide technology, applied in the direction of lithium oxide;/hydroxide, etc., can solve the problems of lack of process conditions and process parameters of waste lithium-ion battery, achieve high comprehensive utilization rate, low environmental protection pressure, short process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: A kind of method utilizing waste lithium ion battery to prepare lithium hydroxide
[0033] Include the following steps in order:
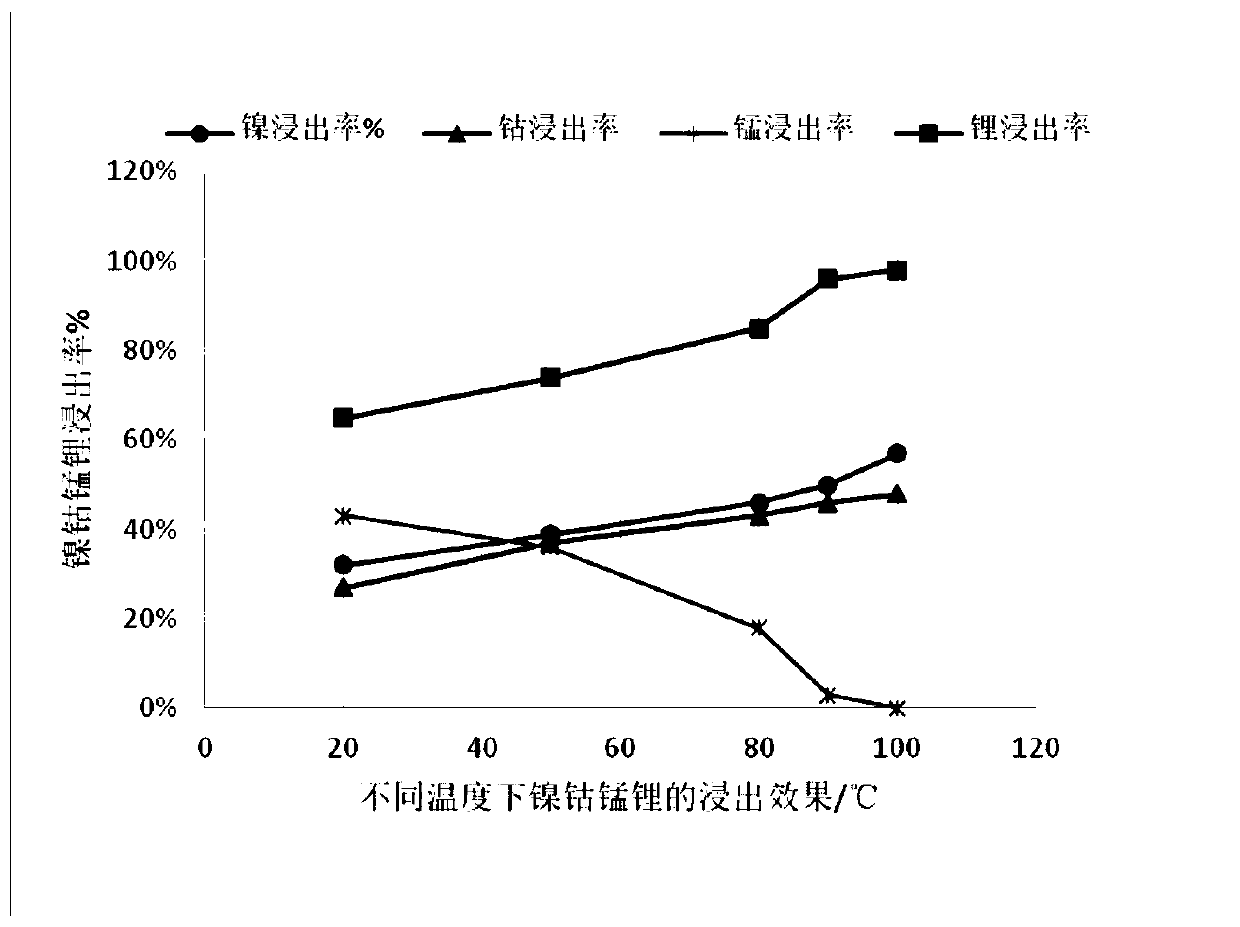
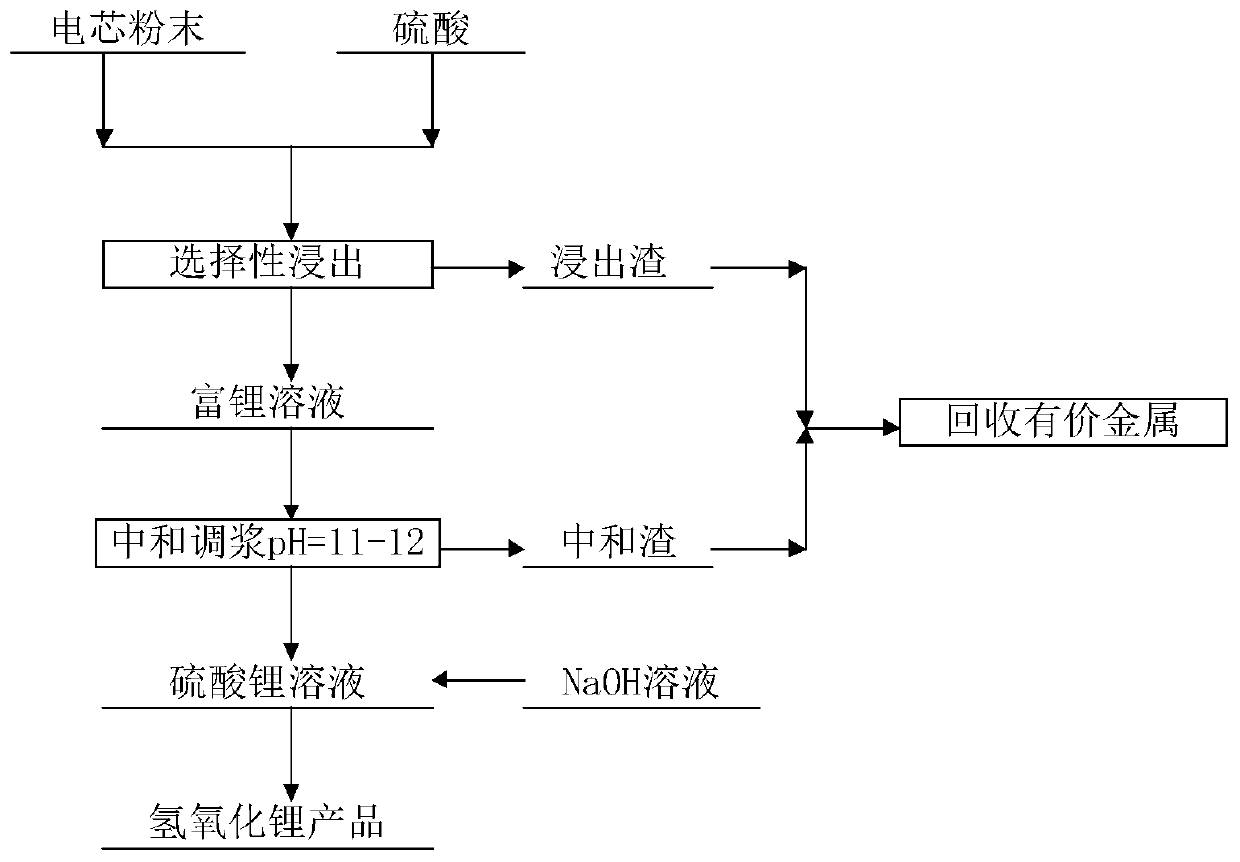
[0034] Take 50g of ternary waste lithium battery cell powder, add 4mol / l sulfuric acid, stir and react at 40°C for 90min, then filter to obtain leaching residue and leaching solution containing nickel-cobalt-manganese-lithium (leaching rate see attached figure 1 ); add sodium hydroxide to the leaching solution, adjust the pH ≈ 11~12, and filter to obtain neutralization slag and lithium sulfate solution.
[0035] According to 0.8 times of the molar theoretical amount of lithium ions, add sodium hydroxide to the purified lithium sulfate solution to obtain sodium sulfate crystals and lithium hydroxide solution; continue to evaporate the lithium hydroxide solution until its solid content reaches 45 to 50 %, cooling to obtain the crude product of lithium hydroxide, and then by means of dissolution-recrystallization, the battery-gra...
Embodiment 2
[0037] Embodiment 2: A kind of method utilizing waste lithium ion battery to prepare lithium ion sieve
[0038] Include the following steps in order:
[0039] Take 50g of ternary waste lithium battery cell powder, add 4mol / l sulfuric acid, stir and react at 60°C for 120min, then filter to obtain leaching residue and leaching solution containing nickel-cobalt-manganese-lithium (leaching rate see attached figure 1 ); add sodium hydroxide to the leaching solution, adjust pH ≈ 11-12, and filter to obtain neutralization slag and lithium sulfate solution.
[0040] According to 1 time of the molar theoretical amount of lithium ions, add sodium hydroxide to the purified lithium sulfate solution, and cool to -8°C under stirring conditions to obtain sodium sulfate crystals and lithium hydroxide solution; continue to evaporate the lithium hydroxide solution, Until its solid content reaches 45-50%, cool to obtain a crude product of lithium hydroxide, and then by means of dissolution-recr...
Embodiment 3
[0041] Example 3: A method for preparing lithium-ion sieves using waste lithium-ion batteries
[0042] Include the following steps in order:
[0043] Take 50g of ternary waste lithium battery cell powder, add 4mol / l sulfuric acid, stir and react at 80°C for 60min, then filter to obtain leaching residue and leaching solution containing nickel-cobalt-manganese-lithium (leaching rate see attached figure 1 ); add sodium hydroxide to the leaching solution, adjust pH ≈ 11-12, and filter to obtain neutralization slag and lithium sulfate solution.
[0044] According to 1.1 times of the molar theoretical amount of lithium ions, sodium hydroxide is added to the purified lithium sulfate solution, cooled to -5°C under stirring conditions, to obtain sodium sulfate crystals and lithium hydroxide solution; continue to evaporate the lithium hydroxide solution, Until its solid content reaches 45-50%, cooling to obtain a crude lithium hydroxide product, and then by means of dissolution-recryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com