Preparation method and application of amido carboxylic acid compound
A technology of amido carboxylic acids and compounds, which is applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problems of poor selectivity, weak collection capacity, and limited development, and achieve short reaction time, The effect of high molecular concentration and reduced energy cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 20.44g lauric acid (content is 98%) and 20.84gN, N'-dicyclohexylcarbodiimide (DCC) (content is 99%) add ball mill jar, grind 30min, then add 14.86g glutamic acid (content 99%) and 8.42g sodium bicarbonate, after continuing to grind for 45min, a white solid product was obtained, which was the target collector product. After analysis and detection, the content of 2-lauroyl amido glutaric acid in the collector product is 52.12%, and the yield of 2-lauroyl amido glutaric acid based on lauric acid is 96.28%.
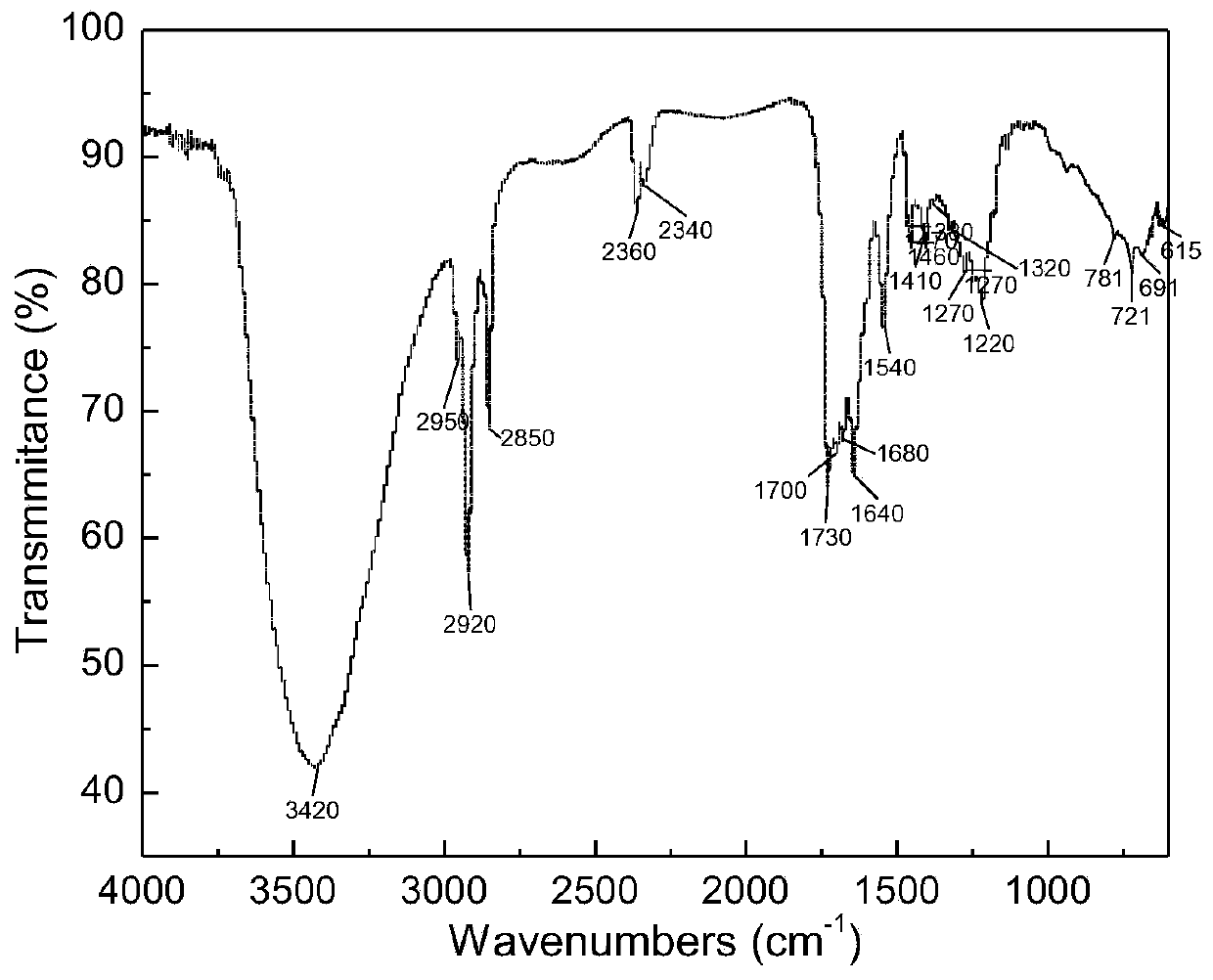
[0063] The product was characterized after being separated and purified by column chromatography, and the infrared spectrum of 2-lauroylamino glutaric acid was as follows: figure 1 As shown, its main characteristic peaks are (cm -1 ): 3420 belongs to N-H stretching vibration peak; 2950 belongs to CH 3 Stretching vibration peak; 2920, 2850 belong to CH 2 Stretch vibration peak; 1700 belongs to C=O stretch vibration peak.
[0064] The mass spectrum of 2-laurylaminogl...
Embodiment 2
[0066] 20.44g lauric acid (content is 98%) and 5.26g acetic anhydride (content is 97%) are added ball mill jar, grind 30min, then add 14.86g glutamic acid (content is 99%) and 8.42g sodium bicarbonate, continue After grinding for 30 minutes, a white solid product was obtained, which was the target collector product. After analysis and detection, the content of 2-lauroyl amido glutaric acid in the collector product is 65.82%, and the yield of 2-lauroyl amido glutaric acid based on lauric acid is 94.89%.
Embodiment 3
[0068] Add 20.44g lauric acid (98% content) and 10.42g N,N'-dicyclohexylcarbodiimide (DCC) (99% content) and 5.78g anhydrous calcium chloride (96% content) to the ball mill tank, grind for 30min, then add 14.86g of glutamic acid (99%) and 8.42g of sodium bicarbonate, and continue grinding for 30min to obtain a white solid product, which is the target collector product. After analysis and detection, the content of 2-lauroyl amido glutaric acid in the collector product is 54.95%, and the yield of 2-lauroyl amido glutaric acid based on lauric acid is 92.71%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com