Improved post-treatment method of valsartan reaction mixed liquid
A mixed liquid and valsartan technology, which is applied in the field of synthesis of drugs and drug intermediates, can solve the problems of copper and other heavy metal explosions, toxic azide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
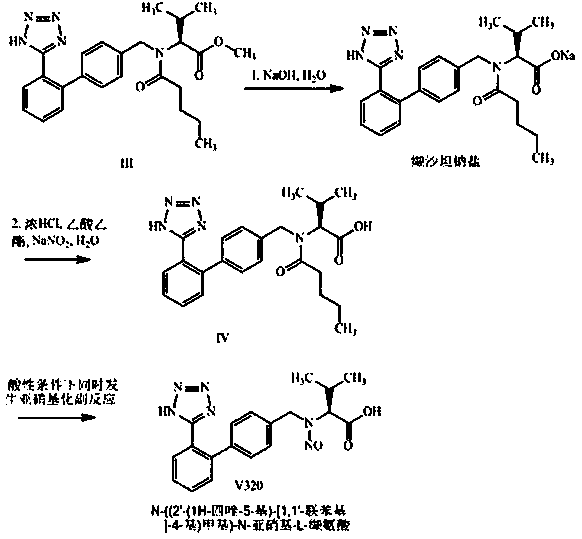
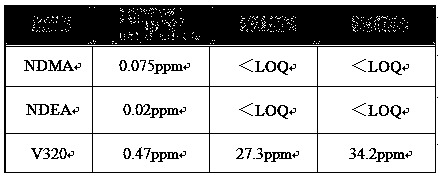
[0037]An improved post-treatment method for the valsartan reaction mixture: add 219.8Kg (540.6mol) N-[(2'-cyanobiphenyl-4-yl)methyl]-N-(1 -Oxopentyl)-L-valine methyl ester (Ⅱ) concentrate (HPLC: N-[(2'-cyanobiphenyl-4-yl)methyl]-N-(1-oxopentyl base)-L-valine methyl ester content ≥ 97.0%), add 200.0Kg diethylene glycol dimethyl ether, control the temperature in the reactor below 45.0°C, and add 132.0Kg (2030.8mol) azidation in batches Sodium and 105.0Kg anhydrous zinc chloride, heat up to 125.0~128.0°C and keep warm for about 35.0 hours, HPLC detects N-[(2'-cyanobiphenyl-4-yl)methyl]-N-(1-oxo Substituent pentyl)-L-valine methyl ester (II) residue ≤ 4.0%, stop the reaction, lower the temperature to below 60.0°C, then add 1800Kg of newly prepared 8.7% (w / w) sodium hydroxide aqueous solution, and stir well , control the temperature of the reaction kettle at 35.0~40.0°C and stir for about 5~6h, the HPLC detection of valsartan methyl ester (Ⅲ) residual ≤ 2.0%, stop the reaction, lo...
Embodiment 2
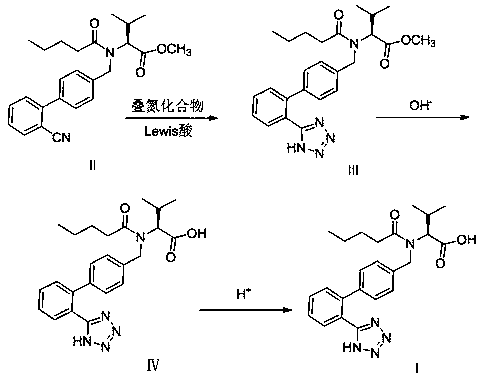
[0040] An improved post-treatment method for the valsartan reaction mixture: in a 500mL clean and dry four-necked reaction flask, add 30.0g (73.8mmol) N-[(2'-cyanobiphenyl-4-yl)methanol Base]-N-(1-oxopentyl)-L-valine methyl ester (Ⅱ), 60.0g toluene and 19.4g (69.3mmol) zinc azide tetramethylethylenediamine double salt [TMEDA· Zn(N 3 ) 2 ] (water content 5.0%, prepared according to Example 1-1 of WO2012148148A2), stir evenly at room temperature, raise the temperature to 105.0-110.0°C, keep warm for 48 hours, the sampling test is qualified, the temperature is lowered to less than 60.0°C, and 45.0g of 30.0% hydrogen is added Sodium oxide aqueous solution and 170.0g water, temperature control 35.0~40.0°C for hydrolysis reaction, heat preservation for 3.0~5.0h, sampling test is qualified, separate toluene layer, the water layer is cooled to less than 20.0°C, add 4.9g (71.0mmol) nitrous acid Sodium, stir evenly, slowly add dropwise 36.0% concentrated hydrochloric acid to adjust th...
Embodiment 3
[0043] An improved post-treatment method for the valsartan reaction mixture: in a 500mL clean and dry four-necked reaction flask, add 30.0g (73.8mmol) N-[(2'-cyanobiphenyl-4-yl)methanol Base]-N-(1-oxopentyl)-L-valine methyl ester (Ⅱ), 60.0g toluene and 19.4g (69.3mmol) zinc azide tetramethylethylenediamine double salt [TMEDA· Zn(N 3 ) 2 ] (water content 5.0%, prepared according to Example 1-1 of WO2012148148A2), stir evenly at room temperature, raise the temperature to 105.0-110.0°C, keep warm for 48 hours, the sampling test is qualified, the temperature is lowered to less than 60.0°C, and 45.0g of 30.0% hydrogen is added Sodium oxide aqueous solution and 170.0g water, temperature control 35.0~40.0°C for hydrolysis reaction, heat preservation for 3.0~5.0h, sampling test is qualified, separate toluene layer, water layer is cooled to less than 20.0°C, add 5.8g (84.1mmol) nitrous acid Sodium, stir evenly, slowly add dropwise 36.0% concentrated hydrochloric acid to adjust the pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com