Method for separating and purifying sulodexide crude drugs from heparin by-product
A technology of heparin by-products and raw materials, applied in the field of medicine, can solve problems such as high production costs, large internal structure damage, and low pass rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
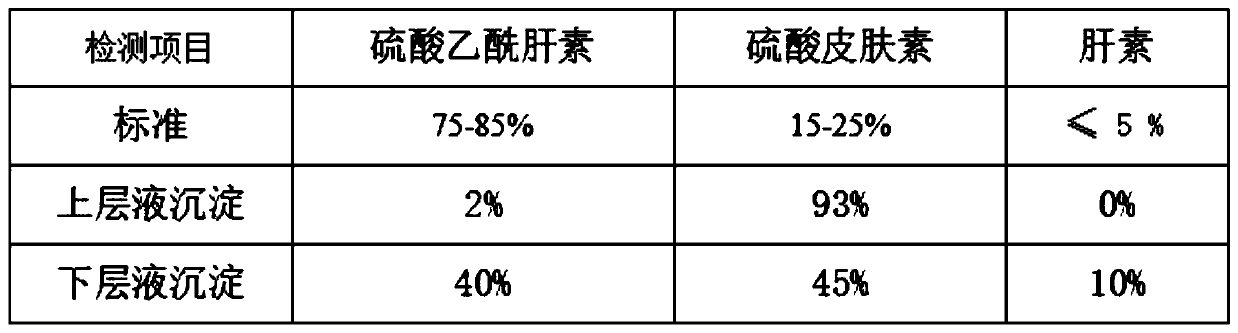
[0047] S1. Pretreatment of heparin by-products: Dissolve heparin by-products heparin 5%, heparan sulfate 45% and dermatan sulfate 47% with a titer of 29 usp u / mg into a solution with a mass fraction of 5%. Dissolve 2% sodium chloride, then add 95% ethanol until the ethanol concentration is 30%, keep it warm for 4 hours, pour out the supernatant, and remove the lower precipitate;
[0048] S2. Separation of heparin: continue to add 95% ethanol to the supernatant obtained in S1 until the ethanol concentration is 50%, keep it warm for 4 hours, precipitate, dehydrate, dry and dry to obtain the crude product after removing heparin;
[0049] S3. Pretreatment of crude heparin: add water to dissolve the crude product in step S1 into a solution with a mass fraction of 10%, raise the temperature to 25° C., dissolve the feed solution with 2% sodium chloride, and then adjust the pH to 11 with sodium hydroxide solution. Then add 2% anhydrous sodium carbonate-sodium bicarbonate buffer soluti...
Embodiment 2
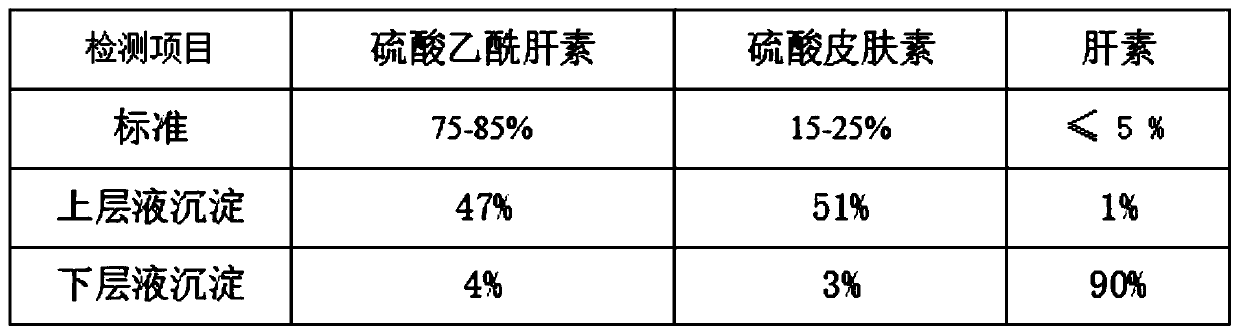
[0053] S1. Pretreatment of heparin by-products: Dissolve heparin by-products heparin 5%, heparan sulfate 45% and dermatan sulfate 47% with a titer of 29 usp u / mg into a solution with a mass fraction of 10%, and heat up to 38°C. Dissolve 2% sodium chloride, then add 95% ethanol until the ethanol concentration is 35%, keep warm for 4 hours, pour out the supernatant, and remove the lower precipitate;
[0054] S2. Separation of heparin: continue to add 95% ethanol to the supernatant obtained in S1 until the ethanol concentration is 50%, keep it warm for 4 hours, precipitate, dehydrate, dry and dry to obtain the crude product after removing heparin;
[0055] S3. Pretreatment of crude heparin: add water to dissolve the crude product in step S1 into a solution with a mass fraction of 10%, raise the temperature to 27°C, dissolve the feed solution with 2% sodium chloride, and then adjust the pH to 11 with sodium hydroxide solution. Then add 2% anhydrous sodium carbonate-sodium bicarbon...
Embodiment 3
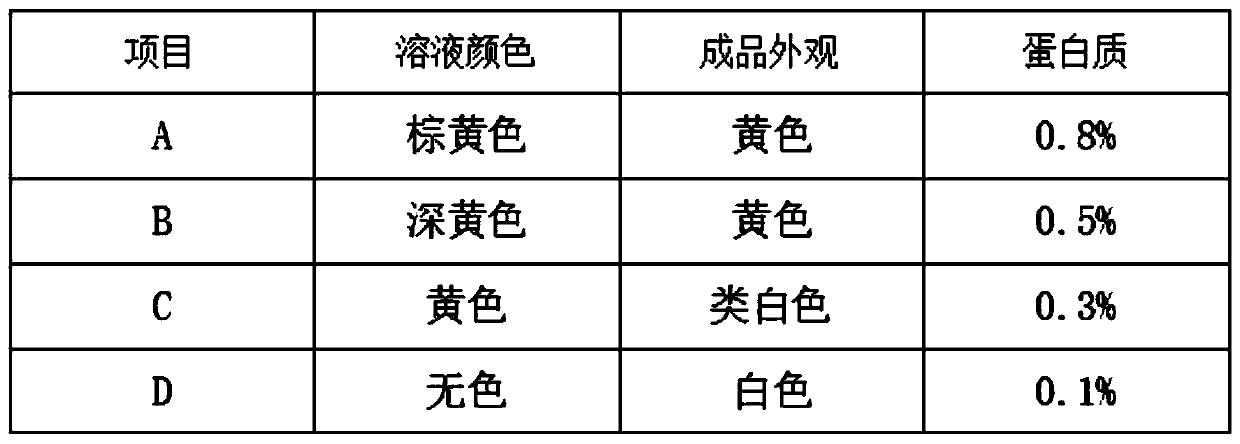
[0059] S1. Pretreatment of heparin by-products: Dissolve heparin by-products heparin 5%, heparan sulfate 45% and dermatan sulfate 47% with a titer of 29usp u / mg into a solution with a mass fraction of 15%, and heat up to 40°C. Dissolve 2% sodium chloride, then add 95% ethanol until the ethanol concentration is 30%, keep it warm for 4 hours, pour out the supernatant, and remove the lower precipitate;
[0060] S2. Separation of heparin: continue to add 95% ethanol to the supernatant obtained in S1 until the ethanol concentration is 46%, keep it warm for 4 hours, precipitate, dehydrate, dry and dry to obtain the crude product after removing heparin;
[0061] S3. Pretreatment of crude heparin: add water to dissolve the crude product in step S1 into a solution with a mass fraction of 12%, raise the temperature to 30°C, dissolve the feed solution with 2% sodium chloride, and then adjust the pH to 11 with sodium hydroxide solution. Then add 2% anhydrous sodium carbonate-sodium bicarb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com