Peramivir solution type inhalant and preparation method thereof
An inhalant and solution-type technology, applied in the field of medicine, can solve problems such as poor stability, achieve good stability, reduce the production of irrelevant substances, and evenly distribute the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
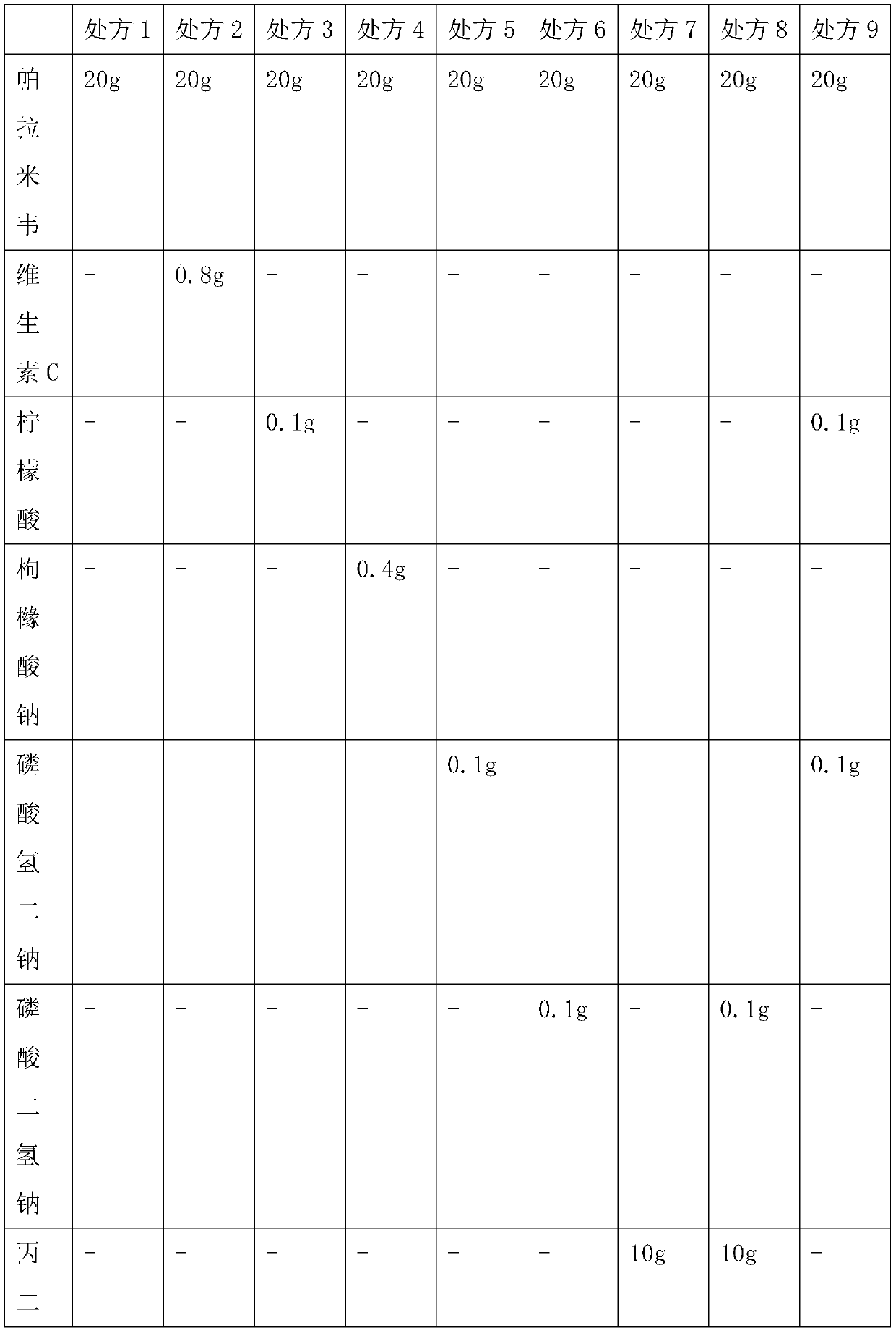
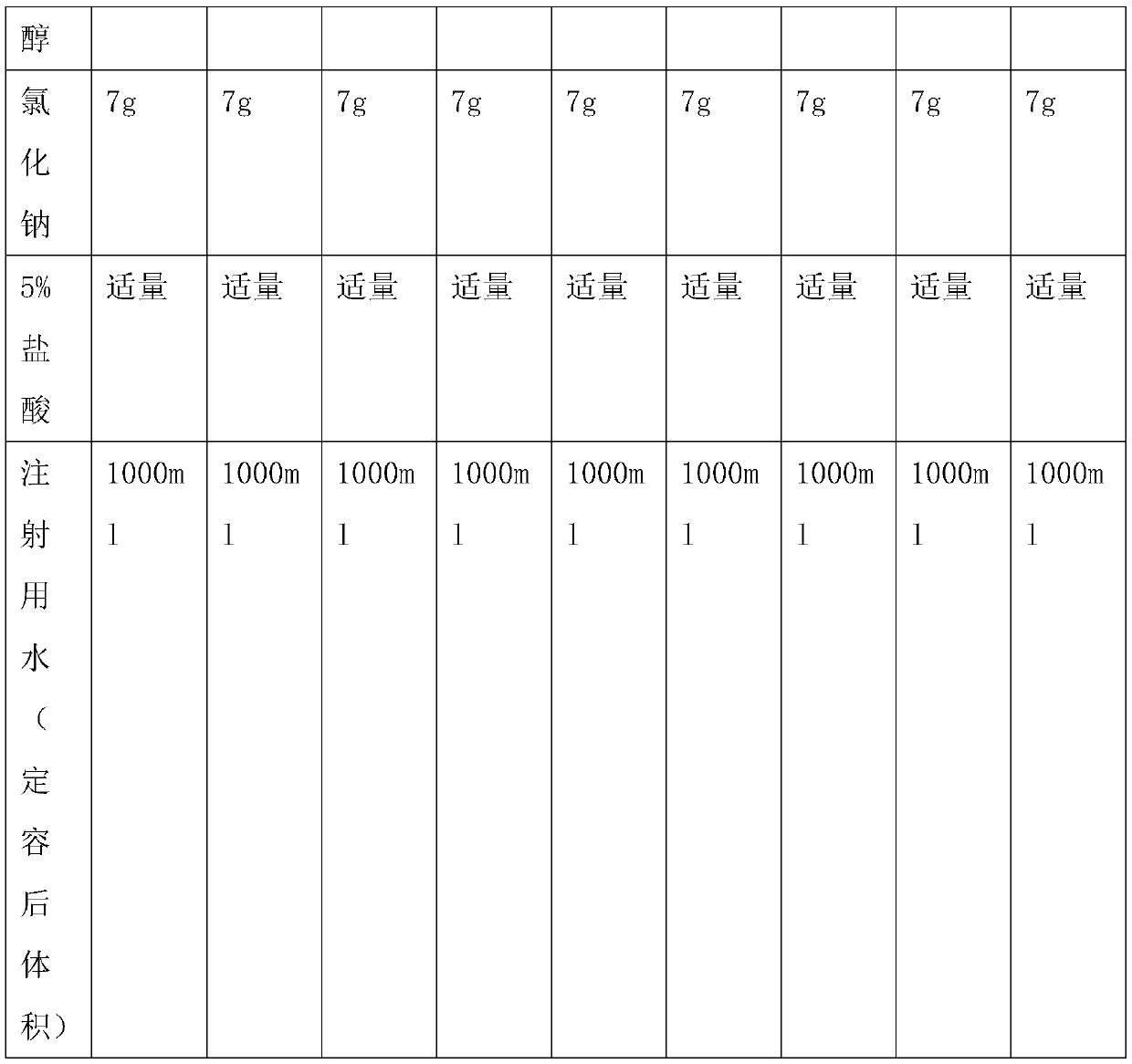
[0031] Example 1: Inhalant Prescription
[0032] According to the prescription in Table 1, mix the peramivir raw material and auxiliary materials in the liquid preparation tank, add water for injection at 70-80°C to 1000ml, stir and dissolve, then cool the solution, and dilute the solution with dilute hydrochloric acid (5% v / v) The pH value is adjusted between 5.0 and 6.0, and then the peramivir solution is pre-filtered and then aseptically filtered, and it is obtained after aseptic filling.
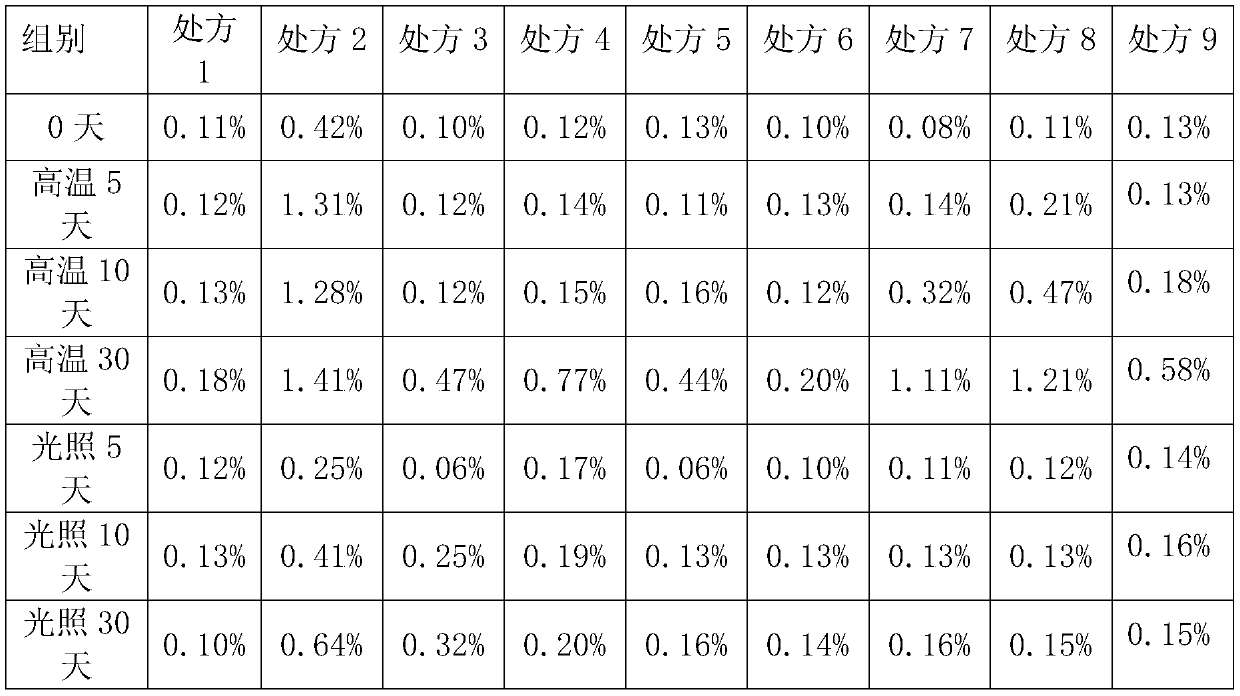
[0033] Prescriptions 1-9 in Table 1 were respectively placed under airtight high temperature (60°C) and light (4500lx ± 500lx) conditions for 0 days, 5 days, 10 days, and 30 days to investigate the situation of related substances.
[0034] Determination method of related substances: HPLC: Agilent 1260Infinity USA; Chromatographic column: EclipsePlus C 18 (5μm, 4.6×250mm, Agilent, USA); mobile phase: gradient elution is as follows:
[0035] Table 1 Mobile phase gradient elution
[0036...
Embodiment 2
[0044] Embodiment 2: Experiment on the influence of different pH on the solubility of peramivir
[0045] Take by weighing excessive peramivir crude drug and add in the 25ml volumetric flask, be settled to scale with 0.7% saline, be placed in shaker shaking (25 ℃, 75rpm) after 24h, with dilute hydrochloric acid (5%, V / V) adjust the pH to 4, 4.5, 5.0, 5.5 respectively, continue to stand for 48h, filter with a 0.45 μm filter membrane, and the filtrate is diluted 250 times with deionized water, and the sample adopts the HPLC method in the peramivir standard to measure the peramivir content .
[0046] Table 4 The equilibrium solubility of peramivir in different pH physiological saline solutions
[0047]
[0048] The experimental results show that the equilibrium solubility of peramivir is pH-dependent, and its equilibrium solubility increases as the pH decreases. Combined with factors such as the requirement (3-8) of preparation pH and physiological stimulation, the pH of per...
Embodiment 3
[0049] Embodiment 3: Stability experiment of peramivir inhalation solution under different pH conditions
[0050] Adjust the pH value of the solution to 4.5, 4.5, 5.0, 5.5 with dilute hydrochloric acid (5% v / v) for prescription 1 and 6 respectively, and the solution that was not adjusted with dilute hydrochloric acid, and then place the peramivir solution in an airtight container Place it under the conditions of high temperature (60°C) and light (4500lx±500lx) for 0 day and 30 days to investigate the situation of related substances.
[0051] Related substance content (%) under different pH conditions of table 5
[0052]
[0053] The experimental results show that the peramivir inhalation solution is the most stable at a pH value of 5.5, and can still maintain good stability under high temperature conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com