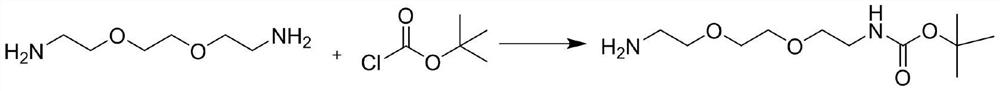A kind of synthesis technique of boc-1-amino-3,6-dioxa-1,8-octanediamine
A synthesis process and dioxa technology are applied in the preparation of carbamic acid derivatives, the preparation of organic compounds, organic chemistry and other directions, which can solve the problems of long steps, long process routes, and total yield of less than 50%, and reach the reaction time. The effect of short, simple reaction route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthesis technique of Boc-1-amino-3,6-dioxa-1,8-octanediamine, it comprises the steps:
[0027] Add 148.2g DODA and 740mL acetonitrile into a 2L reaction flask, stir and lower the temperature to 0-5°C, dissolve 150.3g (1.1 equivalent) tert-butoxyyl chloride in 445mL toluene, and then control the temperature at 0-5°C. The toluene solution of tert-butoxyacyl chloride is slowly added dropwise to the acetonitrile solution of DODA, and the time of dropping is controlled within 1-2 hours. After the dropwise addition, stir for 30 minutes, filter, and filter the cake with a mixture of 70 mL of acetonitrile:toluene=5:3. Solvent rinsing, the filter cake after rinsing was dissolved in 1L of water, adjusted to pH 10 with sodium hydroxide solution, extracted twice with methyl tert-butyl ether, 750mL each time, combined organic phases, washed with 250mL of water, concentrated , and dried to obtain 183.7g of Boc-1-amino-3,6-dioxa-1,8-octanediamine with a purity of 99.3% and...
Embodiment 2
[0029] A kind of synthesis technique of Boc-1-amino-3,6-dioxa-1,8-octanediamine, it comprises the steps:
[0030] Add 148.2g DODA and 740mL acetonitrile into a 2L reaction flask, stir and cool down to 0-5°C,
[0031]Dissolve 136.6g (1.0 equivalent) of tert-butoxyyl chloride in 445mL of acetonitrile, then slowly add the acetonitrile solution of tert-butoxyyl chloride dropwise to the DODA acetonitrile solution at a temperature of 0-5°C, and the dropping time is controlled at 1 -2 hours, after the dropwise addition, stir for 30min, filter, filter the cake with 70mL of acetonitrile: methyl tert-butyl ether = 5:3 mixed solvent rinse, the filter cake after the rinse is dissolved in 1L of water and then oxidized with hydrogen Adjust the pH of the sodium solution to 10, then extract twice with methyl tert-butyl ether, 750 mL each time, combine the organic phases, wash with 250 mL of water, concentrate, and dry to obtain 131.6 g of Boc-1-amino-3,6-bis Oxa-1,8-octanediamine, the purity...
Embodiment 3
[0033] A kind of synthesis technique of Boc-1-amino-3,6-dioxa-1,8-octanediamine, it comprises the steps:
[0034] Add 148.2g DODA and 740mL toluene into a 2L reaction flask, stir and cool down to 0-5°C. Dissolve 150.3g (1.1 equivalent) of tert-butoxyyl chloride in 445mL of toluene, then slowly add the toluene solution of tert-butoxyyl chloride dropwise to the toluene solution of DODA at a temperature of 0-5°C, and the dropping time is controlled at 1 -2 hours, after the dropwise addition, stir for 30 minutes, filter, rinse the filter cake with 70mL of toluene, dissolve the rinsed filter cake with 1L of water, adjust the pH to 10 with sodium hydroxide solution, and then use methyl tert-butyl Extracted twice with ether, 750mL each time, combined the organic phases, washed with 250mL of water, concentrated, and dried to obtain 74.5g of Boc-1-amino-3,6-dioxa-1,8-octanediamine with a purity of 95.3% , yield 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com